Clinical Evaluation and Investigation of Medical Devices under the new EU-Regulation
This page provides the ressources for the book Clinical Evaluation and Investigation of Medical Devices under the new EU-Regulation.
ORDER BOOK HERE
The information on this page is intended to be read in conjunction with the textbook and is structured as follows:
Foreword
“I first met Wolfgang (Ecker) in 2015, having moved from working as a busy junior doctor in an Irish hospital to working as a medical officer for medical devices for my national Authority.
In my second week on the job, I was asked to attend a meeting in Brussels to discuss what became Annex VII of the Medical Device Regulation. I still remember being introduced to Wolfgang and I was immediately struck by his kind and enthusiastic manner.
Wolfgang was a key figure in representing the importance of clinical evidence as part of the European Council Working Party negotiations for the Medical Device Regulation. At the Clinical Investigation and Evaluation Working Group, Wolfgang also worked very hard to ensure progress the last revision of the guidance for clinical evaluation (MEDDEV 2.7/1 revision 4), in addition to a range of other technical achievements.
In Europe, you learn very quickly that achieving progress requires teamwork, consultation and effective communication.
This textbook represents a thorough and detailed guidance, with many practical and useful elements to help medical device developers to meet the requirements of the new Regulation.
When I speak about medical device regulations, I often find myself explaining the differences between medical devices and medicinal products, in terms of the numbers of products, the decision makers for market access, the legal standard for clinical evidence, but also the differences of profiles of drug and device developers. Medical devices are often developed in smaller institutions, whether ‘spin-out’ companies or small and medium sized enterprises. As such, a thorough and practical textbook explaining the requirements and providing practical solutions is an important addition to the available knowledge and I congratulate Wolfgang and the fellow authors for their achievement.“
Dr. Tom Melvin Co-Chair, Clinical Investigation and Evaluation Working Group
Introduction
Both in the old and new EU regulatory system for medical devices (MD), the manufacturer has to demonstrate safety and performance of MDs not only by technical and preclinical evaluation but also by clinical evaluation on the basis of sufficient and relevant clinical data. One of the main challenges for clinical evaluators is to adequately reflect the complex character of the clinical evaluation, ranging from regulatory, organizational, technological to clinical aspects. Subsequently, the expectations on the documentation of the clinical evaluation are manifold and in large parts related to the perspective of evaluators serving manufacturers or third parties (National Competent Authorities, Notified Bodies, Public…). This complexity is additionally increased since MDR is explicitly placing clinical evaluation as an active systematic life cycle process under the manufacturer's mandatory QMS.
From a regulatory perspective: In order to assure that the expectations, claims and requirements concerning clinical safety and effectiveness are fulfilled in the intended target population(s) and indications, the manufacturer must generate, identify, appraise, analyse, evaluate, document and update sufficient and methodologically valid clinical data over the life-cycle of the MD (including PMCF ) and demonstrate this as the clinical evidence in conjunction with the clinical evaluation report (CER), following a proper process of clinical evaluation.
From an organizational perspective: The improvement of the clinical evaluation (CEV) of MDs has been one of the main target areas of the new medical device regulation (MDR). MDR is explicitly placing clinical evaluation as an active systematic life cycle process under the manufacturer's mandatory QMS. Clinical evaluation is now more closely integrated into the systemic context of the new regulation, especially with regard to its connections to QMS, PMS, risk management, manufacturer’s obligations (Art. 10), demonstration of conformity with the general requirements for safety and performance (Annex I), technical documentation (Annexes II and III), tasks and competencies of notified bodies (Annex VII) and to conformity assessment (Annexes IX-XI ). This also implies that the interconnections to other processes have to be established and be continuously evaluated. This integration into the quality management system requires that the responsible persons involved are adequately qualified - a respective rationale also needs to be provided if (parts of) the process is outsourced. These aspects can be seen as the organizational framework for the activities related to the clinical evaluation.
From a technological perspective it is expected that the device description within the clinical evaluation report correctly identifies the current configuration of the medical device, including (but not limited to) the name, model, sizes, variants, components of the device (including software, accessories or intended product combinations). Clinical evaluators have to critically rely on previous technical and preclinical evaluation, based on concise physical, chemical, technical specifications and mechanical and functional characteristics and a clear understanding of the mode of action. Further on, the technological and biological differences between predecessor devices and potential equivalent devices must be precisely described and further discussed from a clinical perspective.
From this clinical perspective it is additionally expected that the clinical data supports the initial and continuous evaluation of the acceptability of the clinical benefit-risk ratio with respect to the current state of the art in medicine, including applicable clinical standards and guidance documents, scientific information regarding the medical condition managed, its natural course and the medical alternatives to the target population . This requires – relating to the device, its technology and its clinical application - deep, usually scientifically based knowledge and critical expertise.
It cannot be emphasized enough, that – due to the necessity of different perspectives - the clinical evaluation process typically cannot be conducted by one single person and in isolation from other processes. An effective implementation of the clinical evaluation requires deep and fundamental changes within the organizational structure of medical device manufacturers and a close interaction with the life-cycle processes of the device.
For clinicians and related scientists, the new EU medical device system offers excellent opportunities, based on their professional know-how and on their knowledge of regulatory, normative and scientific principles and processes.
This applies both if you want to cooperate with the stakeholders (above all manufacturers, notified bodies or competent authorities) and even more if you want to make your own involvement stand out in start-ups, spin-offs or other development projects or in counselling services. The increased emphasis on clinical aspects in the MDR is also reflected in the creation of an improved clinical infrastructure of the EU regulatory system with (clinical) expert panels and the provisions for product group-specific clinical guidelines as “Device Specific Guidance” (DSG ) or in the form of implementing acts of the COM as Common Specifications (CS ) for clinical investigations and/or clinical evaluation and/or PMCF of certain types or groups of (usually high-risk) MDs. The clinical aspects are now more clearly highlighted in the terminology (Art.2 of MDR). Clinical Evaluation and its assessment is now clearly at the centre of the manufacturer’s and notified bodies obligations; Clinical Evaluation and its assessment has to be properly set up, documented and updated. Its results will be more transparent to the public .
The new EU medical device regulatory system now needs significantly more competence for Clinical Evaluation and its assessment!
Book Content
1. CLINICAL EVALUATION
1.1. Introduction
1.2. Clinical Evaluation - Definitions
1.3. History of Clinical Evaluation within the EU Regulatory System for medical devices
1.4. The legal basis for Clinical Evaluation within the MDR
1.5. Clinical Benefit/Risk determination and the Clinical Evaluation process and its steps
1.6. Clinical Evaluation Process – The Steps
2. SYSTEMATIC SEARCHING FOR LITERATURE ON CLINICAL (STATE OF THE ART) EVIDENCE OF MEDICAL DEVICES
2.1. Checklist to support efficient information retrieval on Medical Devices Evidence
2.2. Planning a Search using the PICO framework
2.3. Developing a literature search protocol (according to MEDDEV 2.7/1 rev 4, A5.3.)
2.4. Scientific (medical) databases for retrieving clinical evidence: An overview
2.5. Efficient search strategies for identifying Clinical Trials on Medical Devices
2.6. The literature search report
3. CLINICAL INVESTIGATIONS WITHIN THE CONTEXT OF THE MDR
3.1. Sources
3.2. Definitions
3.3. Overall Aspects of Clinical Investigations under the MDR
3.4. Clinical Development Stage
3.5. Clinical Investigation Plan (CIP)
3.6. Statistical Considerations
3.7. Investigators Brochure (IB)
3.8. Clinical Investigation Report (CIR) and its Summary Report
3.9. Informed Consent and Special Protection of Vulnerable Subjects/Populations
4. MONITORING OF CLINICAL INVESTIGATIONS OF MEDICAL DEVICES FOR HUMAN SUBJECTS
4.1. Sources
4.2. General Requirements for Monitoring
4.3. The Monitor
4.4. Assessment of the Investigation Site
4.5. Initiation of the investigation Site
4.6. Routine monitoring visits
4.7. Close-out Visits
4.8. Monitoring reports
4.9. Supporting Documents
Authors
Hon (FH) Prof.Dr. Wolfgang Ecker, MD, graduated at the Medical Faculty of the Uni-versity of Vienna, has accomplished his medical training as GP in various Viennese hospitals. He has served the Austrian Health Ministry and the EU Medical Device Sector for 30 years. He has been member of various expert groups at EU level, i.a. as Chair of the EU Working Group on Clinical Investigation and Evaluation (CIE) and as an EU representative in GHTF Study Group 5 on Clinical Evidence. As a member of the EU Council Working Group on Medical Devices he has helped shaping the new EU Regu-lations on Medical Devices and IVDs. He is giving lectures and training seminars on these new Regulations at various Universities of Applied Sciences in Austria and at Health Technology Clusters.
Ass. Prof. Dr Gerold Labek, M.D., graduated at the medical faculty of Vienna Univer-sity. He has accomplished training for General Practitioner and senior consultant for orthopedic surgery at AKH Linz (now Kepler University Clinic Linz). He conducted research focused on the topics of registries/real world evidence and quality of pub-lished research for almost 20 years and received habilitation at Innsbruck Medical University on research methodology. He was been working for the Notified Body TÜV SÜD as Director for Clinical Market Surveillance and is active now as clinical expert for several Notified Bodies.
Tarquin Mittermayr, BA(Hons), MA is an Information Specialist at the Ludwig Boltzmann Institute for Health Technology Assessment, where he is responsible for the institute’s information management including the systematic searching for scientific literature. He studied History, Archive and Museum Studies as well as German, Euro-pean Studies and Bookbinding. He worked as an assistant librarian at the monastery of St. Florian, as an Information Assistant at Roehampton University and St. George’s Hospital Medical School (University of London), and more recently as an archivist at the Konrad Lorenz Institute for Evolution & Cognition Research.
Brigitte Raffeiner, PMSc is CEO of the Competence Center for Medical Devices. Her personal focal points in the company are corporate management, project management, monitoring and data management. She can look back on many years of experience in the handling of clinical studies. Together with her partners, she supports companies in successfully bringing their products to market.
DI (FH) Dr. Michael Ring is co-founder and one of the managing directors of R'n'B Consulting GmbH and co-founder and consultant at the Competence Center for Medical Devices (CCMD). Together with his business partners and co-operation partners, he supports companies in the medical technology industry in the compliant implementation of the process of the clinical evaluation, including the conduct of clinical investigations and systematic literature searches. In addition, Michael worked from 2014 to 2019 as a study coordinator at the newly established Kepler University Hospital in Linz. His responsibilities included the coordination of sponsored studies and academic research.
Dr. Bernhard Schwartz, BSc, MSc is a researcher at the University of Vienna and at the University for Applied Sciences for Health Professions Upper Austria as well as a co-founder of Competence Center for Medical Devices GmbH (CCMD). As scientific member and biometrician at an institutional ethics board and laureate of an Austrian State Price for science (Award of Excellence), he - in partnership with the other CCMD members – supports customers in the pharmacological and medical technology in-dustry in planning, execution and statistical analysis of clinical trials. In addition, Bernhard regularly conducts systematic reviews, clinical evaluations and mini health technology assessments (mini HTAs). Based on his former experience in the field of toxicology (Seibersdorf Laboratories) and medical devices (University for Applied Sciences Upper Austria), he has a perfect background for solving clinical and technical problems.
Supporting Material
Chapter 1 - CLINICAL EVALUATION
Rationale (Excerpt from Publication)
It is a well-known fact that certain circumstances during the conduct of a clinical trial, e.g. patient selection, the surgeons’ expertise and experience, or the study design, may have an impact on the results, and the question to what extent the results obtained are reproducible in the total patient population produces on-going critical discussions. This does not only apply to pharmaceutical studies but, of course, also to medical devices such as artificial joint implants. In principle, two major datasets are available for the assessment of implants or surgical techniques:
- Sample-based clinical studies that have been published in scientific journals
- National and regional arthroplasty registers
Each of these datasets is characterised by specific priorities and requirements that should be taken into account in project planning and when interpreting the results.
Sample-based clinical studies:
- Try to extrapolate the results of a sample to the total patient population;
- Are usually applied to answer a particular question;
- Study design, measuring instruments or patient selection are therefore often very non-homogeneous, which is an essential advantage with regard to the pre-cision in tuning the instruments but, in a meta-analysis, may lead to limitations.
- The characteristics of the collected data substantially affect the validity and possibilities of evaluations. Ordinal and nominal data, such as Yes/No decisions, or the formation of groups (for example: Excellent; Good; Fair; Poor) require relatively large numbers of cases in order to produce statistically significant differences and ensure sufficient statisti-cal power. For example, the question of whether revision surgery has been per-formed falls into this category. In his PhD thesis Leif Ivar Havelin has shown that, to comply with the usual standards of a 95% confidence interval and a statisti-cal power of 80%, a prospective study would require 13,474 patients in order to determine a 1% difference in outcome between two implants. Also, it would still require 3,008 patients to detect the rela-tively big difference of two percentage points. The execution of studies of that size quickly reaches organisational limits so that one must conclude by implication that the large majority of published stud-ies might be statistically underpowered. Metric data, on the other hand, as used in most clinical scores, allow for reasonable evaluations already with a considerably lower number of patients.
Registers:
- Are designed to comprise all surgeries performed in a defined region, e.g. a state, thus providing a very realistic picture of the actual circumstances.
- To achieve completeness, the burden of documentation must not be too great. The questionnaires must therefore be confined to a relatively small core dataset.
- Apart from organisational difficulties, any modification to the dataset reduces the value of the data already collected for evaluations. Thus, registers are relatively inflexible.
- Data transferred to the register centre by the individual departments can thus be verified to a very limited extent, which should be taken into account when decid-ing on the contents to be recorded. Only objective and clearly-defined contents should be considered for the core dataset. However, many clinical scores contain a variety of data, such as pain or quality of life, that are strongly affected by subjective influences. These contents are less suited for regular data collection, but can be used successfully in projects including register datasets.
Thus, the two instruments and data sources do not compete with each other, but can sensibly complement one another. Registers offer advantages in recording and evalua-tion as regards revision rates and causes of revision. They are able to provide a realistic picture of the results in the area covered and considerably alleviate or eliminate effects arising in clinical studies, for example, due to patient selection or personal expertise. Completeness of collection is therefore an essential parameter for the quality of a register dataset. Clinical studies, on the other hand, have undeniable advantages in dealing with specific issues and subjectively-influenced answers.
Registers have been developed with great success in Scandinavia for more than 30 years, and impressive proof has been established of their usefulness for outcome measurement, quality control and quality improvement in many cases (1-15). During the past 10 years similar projects have been set up in quick succession in other countries so that an increasing number of datasets have become available for supranational analyses by now. These datasets can be used as reference values for compara-tive analyses regarding the reproducibility of published results. The quality, size, geographical distribution and length of follow-up periods of these datasets are only available in very few areas of medicine and for a very small number of indications. Thus, arthroplasty repre-sents a positive exception in the medical field, and we have taken advantage of these positive circumstances for conducting a fundamental and critical analysis of those basic data that decisions have been based on worldwide.
Revision Rate is a recognised, well-defined and objective parameter after arthroplasty interventions that covers a variety of possible complications. The necessity for revision surgery has serious consequences for the patient’s quality of life and causes high health-care expenditure. Decision-making largely follows standard procedures in diagnostic assessment and indication. This indicator is therefore well-suited for comparative analyses, and the conclusions are relevant for all major parties involved in the health-care system.
Chapter 2 - SYSTEMATIC SEARCHING FOR LITERATURE ON CLINICAL (STATE OF THE ART) EVIDENCE OF MEDICAL DEVICES
PubMed
Cochrane Library
ClinicalTrials.gov - WHO ICTRP - EU Clinical Trials Register
Embase
Chapter 4 - MONITORING OF CLINICAL INVESTIGATIONS OF MEDICAL DEVICES FOR HUMAN SUBJECTS
Disclaimer: The following templates are thought to provide a general guidance for the creation of study-specific templates. We do not guarantee the full compliance with national laws - each document has to be verified and adapted according to the local legal requirements.
ANNEX A - TRANSITION FROM DIRECTIVES TO REGULATIONS
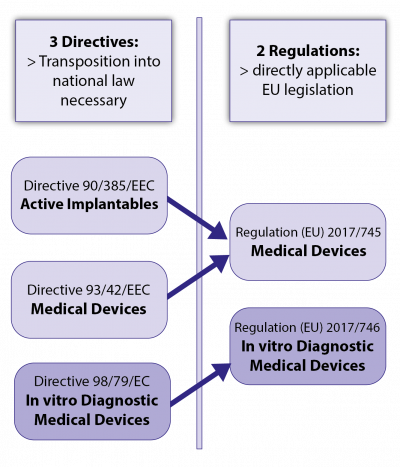
In 2017, the EU legislator transformed the previous set of regulations for medical devices, consisting of 3 EU directives and their national implementations, into a new system of 2 EU regulations as now directly applicable EU law:
- Regulation (EU) 2017/745 on medical devices (MDR), and
- Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR).
Both regulations were developed in parallel and each consist of 3 major parts(see Fig.2):
- Recitals, i.e. intentions and objectives of the EU legislator which can be used as an aid to interpretation in the event of legal uncertainties,
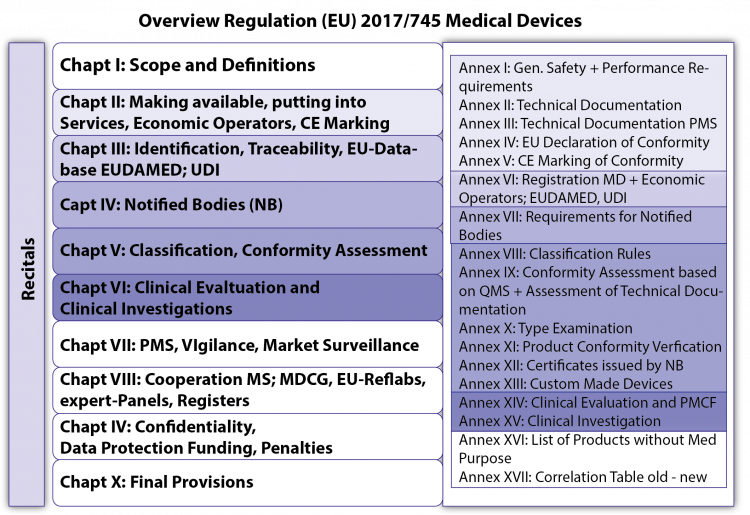
- the Chapters and their Articles, with the core of both legal texts, and
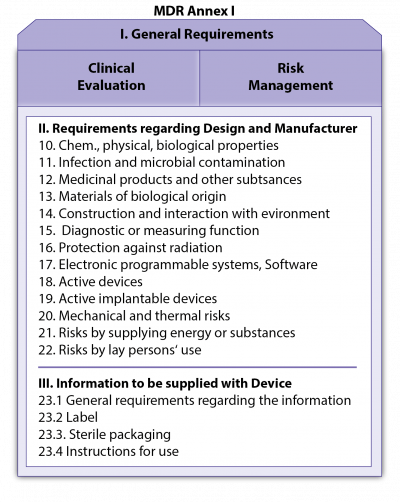
- the Annexes, the binding, more technically oriented legal text, which supplement the chapters (for example Annex I on General Safety and Performance Requirements [GSPR; previously: Essential Requirements – ER], Annex II and III on technical documentation or Annexes IX to XI on conformity assessment modules [= modules of “European Premarket Approval”]).
For the clinical evaluation and clinical investigations of MDs Chapter VI and Annex XIV (clinical evaluation) and Annex XV (clinical investigations) play the most important role.
The MDR/IVDR entered into force at EU level on 25 May 2017; the date of application for the MDR has been foreseen as 26 May 2020 (3-year transitional period), but a proposed amendment to the MDR should postpone that to 26 May 2021; for the IVDR, 26 May 2022 (5-year transitional period) is currently foreseen.
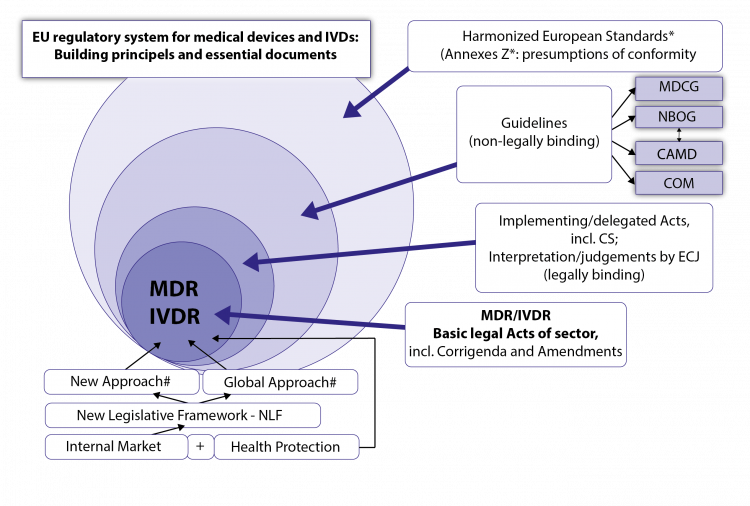
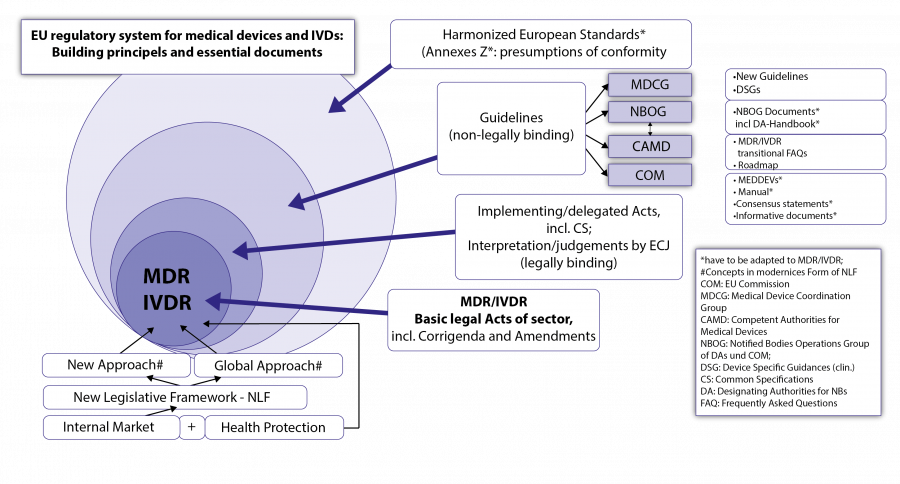
A.2. The New EU Regulatory System for Medical Devices and IVDs - The Building Principles
Both regulations are based on fundamental legal principles of the EU: the internal market concept and health protection (which primarily aims at safety and effectiveness, a high level of health protection, a positive clinical benefit/risk ratio according to the state of the art and minimization of risks and side effects).
A.2.1 New legal framework for EU product legislation
This is about the new general legal framework for (many) EU product regulations (New Legislative Framework - NLF), which constitute the background philosophy of MDR and IVDR. NLF builds in a modernized form on important legal construction principles, which are essential for the understanding of both regulations: These are the
- New Approach and the
- Global Approach.
A.2.2. The New Approach Under the New Legal Framework
The New Approach is essentially about the following: The two Regulations define in their respective Annexes I checklist-like the General Safety and Performance Requirements (GSPR; see Fig. 4; previously called Essential Requirements - ER; at IMDRF and ISO-level also called Essential Principles) of the MDs or IVDs, which, insofar as they apply to a certain MD/IVD, must be fulfilled by the products on placing on the market or on putting into service.
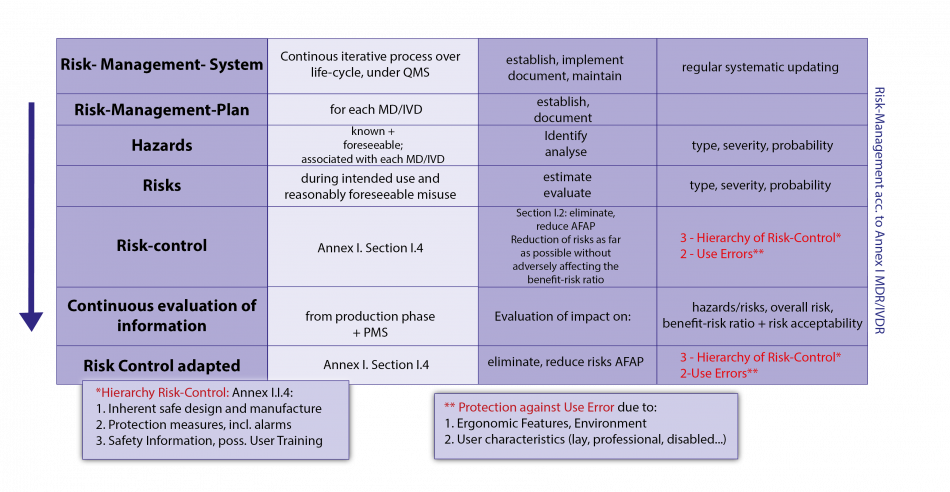
The basic GSPR are clinical evaluation and risk management.
The details to these requirements incl. eventual verification procedures and tests, or on certain processes and procedures of the regulatory system, are provided outside the legal texts by harmonized European standards, the references (titles) of which are published in the Official Journal of the EU for the respective Regulation or Directive. These harmonized standards are not binding , but contain in their Annexes Z (ZA, ZB, …) very specific presumptions of conformity with regard to the GSPR mentioned there for an MD/IVD; i.e. if the manufacturer follows the presumptions of conformity set out in Annexes Z (ZA, ZB,…) of the standards for the fulfilment of certain GSPR, the manufacturer is entitled to a presumption of conformity in this respect from its Notified Body (NB; “European Conformity Assessment Body”) or from the market surveillance authority (this presumption may be falsified under certain conditions). When harmonised European Standards are lacking or outdated, state of the art standards may be used. The manufacturer may deviate from harmonized standards but must then sufficiently justify his alternative solutions for the fulfilment of the relevant GSPR, which is associated with increased work load. Harmonized European standards can also provide presumptions of conformity in important processes and procedures (e.g. quality management systems, clinical investigations, usability, software, risk management). The Harmonised Standards are drawn up by the European standardization bodies CEN and CENELEC (the latter for the electrotechnical sector) on the basis of standardization mandates of the EU Commission with the help of the standardization bodies of the MSs; they are jointly agreed and harmonized for certain regulations or directives after examination by the Commission. Their references (titles) are published 1-2 times a year in the Official Journal of the EU . European standards are usually developed jointly with the global standards institutions ISO (<> CEN) and IEC (<> CENELEC); however, Appendices Z with the presumptions of conformity are only valid for the Harmonized European Standards. Further presumptions of conformity can now be provided for certain MD/IVD groups within the framework of both regulations, especially in the clinical area, by Common Specifications, (CS) in the form of legal acts of the COM.
A.2.3. The Global Approach under the New Legal Framework
The global approach deals with the conformity assessment (“European Premarket Approval”) of products on the basis of modular conformity assessment procedures. The modules for conformity assessment are represented in Annexes IX to XI:
- Annex IX: QMS and Assessment of Technical Documentation
- Annex X: EU Type Examination
- Annex XI: Product Conformity Verification: Part A: Production Quality Assurance or Part B: Product Verification
These must be selected according to the class of the MD/IVD, derived upon the classification rules of Annex VIII of the MDR/IVDR. For medium and higher classes of the MD/IVD, conformity assessment is carried out by so-called Notified Bodies (NB). Notified Bodies are appointed by the MS acc. to their proven qualification and competence in a complex, now European supervised procedure for
- certain product areas,
- certain special aspects (e.g. certain technologies (e.g. nanotechnology) or processes (e.g. molecular biological diagnostics), so-called “horizontal codes”
- certain conformity assessment modules (Annexes IX-XI or parts thereof).
The positive completion of conformity assessment modules (or certain parts thereof) by the manufacturer is documented by certificates (NB-certificates) from the NB (valid for a maximum of 5 years; see Annexes XII in both Regulations). The aim of conformity assessment is the CE marking of conformity as a sign of successful EU approval (the correct CE marking is set out in Annex V of both Regulations). The CE marking not only includes proof of compliance with the requirements of MDR/IVDR but also of all EU legal acts that also apply to the product and require CE marking (“inclusive” CE marking). The CE marking is supplemented by the 4-digit identification number of the NB (if) involved in the conformity assessment . In addition, the manufacturer confirms the conformity of his medical devices/IVDs with the MDR/IVDR and the other EU legal acts applicable to his product, which also require an EU declaration of conformity in addition to the MD/IVDR, in an EU Declaration of Conformity (“inclusive” EU Declaration of Conformity) whose basic conditions are described in Art. 19/17 of the MDR/IVDR and whose minimum contents are given in Annex IV of both regulations .
A.2.3. Plausibility Checks for Successful "European Conformity Assessments" of MD and IVD
These can be based mainly on the following elements: Plausibility Check for Successful European Conformity Assessment
- CE marking,
- EU Declaration of Conformity,
- Identification Number of the Notified Body (4 digits; if one was involved),
- Certificate(s) of the Notified Body(s) (if any) involved,
- Possible control via EUDAMED (future EU databank, if already functional)
A.2.4. ‘Special Routes’ to Market/User
In addition to the main route to CE marking, other access to the market/user without CE marking is possible in defined special routes, such as (humanitarian) device exemptions or emergency use authorisations by MS or COM , in-house production in/for health care facilities in the EU, clinical investigations of MDs or performance studies of IVDs, systems and procedure packs for MD, etc.
A.2.5. Post-Market Surveillance (PMS), Vigilance and Market Surveillance
Manufacturers and Competent Authorities, but also NB and COM have certain obligations after the start of marketing of an MD. Post-Market Surveillance (PMS) (Chapt. VII.1 of MDR): Manufacturer, Competent Authorities
Vigilance (Chapt. VII.2 of MDR): Manufacturer, Competent Authorities
Market Surveillance (VII.3 of MDR): Competent Authorities
The manufacturers PMS is closely related to the PMCF, both as regards PMCF-plan (Annex XIV.B. and PMS-plan (Annex III of MDR), the necessary analysis and evaluations and the subsequent reporting and drawing conclusions for possible CAPAs, FSCAs, changes to documentation (incl. TD, Annexes II+III) and updating of clinical evaluation, PMS and risk management.
A.3. Important Documents of the Regulatory System
A.3.1. The Main Legal Acts for MD and IVD
- Regulation (EU) 2017/745 concerning medical devices (MDR), and
- Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR)
Possible changes to the basic legal acts MDR and IVDR, which may take the form of corrigenda or amendments . These will be published in the Official Journal (OJ) of the EU and will be referenced at the COM’s home-page of the sector. Drafts of amendments or corrections may be seen earlier in official consultation procedures to the public and stakeholders.
Implementing and Delegated Acts of the COM; Judgments by ECJ: Legally binding
Besides the two basic regulations we can expect a wealth of implementing legislation from COM, either as implementing acts or delegated acts based on authorisation in the regulations. This includes the future Common Specifications (CS) with specific requirements, especially for clinical aspects (clinical investigations, clinical evaluation, PMCF of specific types of MD) of certain high-risk product types, but also of Annex XVI products (for aesthetic or life-style purposes). Already issued: COMMISSION IMPLEMENTATION REGULATION (EU) 2017/2185 of 23 November 2017 on the list of codes and corresponding product types for determining the scope of the designation of a Notified Body in the field of medical devices under Regulation (EU) 2017/745 of the European Parliament and of the Council and in the field of in vitro diagnostic medical devices under Regulation (EU) 2017/746 of the European Parliament and of the Council. (This will be important for the search of suitable NBs!) Further implementation legislation will follow; the prioritization can be anticipated on the basis of the roadmap on the CAMD homepage and the rolling plan of the COM ; within the framework of regulatory compliance, these must be tracked under the QMS as well as any Amendments and Corrigenda of the underlying MDR and IVDR. The (legally binding) monopoly on interpretation of EU law is the responsibility of the European Court of Justice and Judgements and Decisions of the ECJ must also be carefully pursued.
Non-legally binding Guidance
Due to the strong heterogeneity of the product area, a dense, non-legally binding set of guidelines (guidance) of the COM (called MEDDEVs for Medical Device Guidelines) and the competent authorities of MSs has been developed for the existing Directives. These have been developed and agreed jointly by all stakeholders in various EU working groups and approved under the patronage of the Medical Device Expert Group (MDEG); They will have to be formally and in their content be made fully compatible now with MDR and IVDR.
Guidance documents of the COM are available on the COM homepage in different formats: Old COM Guidance: MEDDEVs (Medical device [and IVD] guidelines ) which have now to be adapted to fit the MDR and IVDR properly and in future will turn into MDCG-Guidance. These cover more or less all areas where (non-binding) legal interpretation concerning the MD- and IVD-Directive(s) and in future also MDR/IVDR will be helpful. The drafts of these guidance documents are generated and further processed in the relevant working groups (in the clinical area specifically WG CIE [Clinical Investigation and Evaluation] now under the MDCG) and may be influenced via stakeholder organisations (industry, health care institutions and professionals, health insurances, patient organisations) participating there.
Other COM guidance formats may in certain cases (presently not in the clinical sector): Consensus statements , and Informative documents. These are also presented at the COM’s homepage.
(new) MDCG documents: Under the MDR/IVDR, new kinds of guidance documents by the Medical Device Coordination Group (MDCG) will be issued. The first series covered UDI-issues and designation criteria for NBs under the new regulations. MDCG Guidance is presented at the COM homepage . The near future work program of MDCG can be seen at the rolling plan of the COM and the CAMD roadmap or the COMs Newsletter for the device sector.
Other insights for anticipation of developments can be gathered via contacts with national competent authorities’ networks (e.g. “Jour fixes”, national mirror groups and newsletters) or stakeholder networks, see Link list.
CAMD Guidance: The Competent Authorities for Medical Devices (CAMD) have so far issued guidance on transitional issues of MDR/IVDR and the roadmap on further development of guidance and other activities under MDR/IVDR. Other insights for anticipation of developments can be gathered via contacts with national competent authorities’ networks (e.g. “Jour fixes” and newsletters) or stakeholder networks, see Link list.
Guidance by MS-Working Groups: NBOG best practice guides, NBOG checklists, NBOG forms: Guidance documents from the Designating Authorities of MS on best practice for NB and the implementation of conformity assessment procedures, including on the assessment of the clinical evaluation of manufacturers. In addition to those for the previous Directives, including the Designating Authorities Handbook, a whole series of documents have already been developed for the designation of the NB under the new Regulations (NBOG documents for Regulation (EU) 2017/745 (MDR) and Regulation (EU) 2017/746 (IVDR)) .
This whole network of (non-legally binding) guidance documents will be revised in the coming years for the purposes of the new EU regulations. The CAMD roadmap and the rolling plan of the COM are the best way to determine the target direction and current priorities. The patronage of many guidelines will in future pass to the MDCG (Medical Devices Coordination Group) and to CAMD. These developments, which are also very important for the manufacturer, should be actively and systematically anticipated and monitored under his QMS for regulatory compliance purposes (change management). See also Fig. 6 EU regulatory system for MDs and IVDs: key documents and construction principles
GHTF/IMDRF Guidance: If you are interested in the global strategic regulatory developments, it might be useful to screen guideline development at IMDRF. These guidelines are all accessible via the IMDRF homepage (also the “old” GHTF guidance). The drafts of forthcoming guidance are presented for global consultation via the various regions, so you might influence them via your region.
Harmonised European Standards
In addition to the Common Specifications (which are implementing legislation of the COM), the Harmonized European Standards (HS) in their Annexes Z also grant presumptions of conformity for certain requirements of the Regulation (and previously the Directives), in particular also the requirements of Annex I (General Safety and Performance Requirements - GSPR). Annexes Z of harmonized European Standards set out in detail which specific sections of the relevant standard provide a presumption of conformity to which general safety and performance requirements or to specific procedures (eg. Clinical investigations of MDs in EN ISO 14155) of the respective Regulation. The lists of Harmonised European Standards (titles only!) for the new Regulations, as well as for the previous Directives, are published 1-2 times a year in the Official Journal of the EU, after review by COM. Note: Standards may also be deharmonised with new findings of unsuitability. The previous harmonised standards must now also be adapted to the new Regulations and harmonised under these again ! These developments must also be closely monitored by the manufacturer under his QMS and must also be taken into account in the context of change management . The harmonised standards are available in their respective language versions from the national standards bodies (usually you will be charged). Other presumptions of conformity may be derived from Common Specifications (CS) or from state of the art standards (in the absence of suitable harmonised standards). Examples of important harmonized European standards for the medical device directives (to be adapted to Regulations!): Please note in the most recent publication in the Official Journal of the EU the current versions (dates) of the standards and the end of the transition period of the outdated previous version; this has to be followed by the manufacturer under the QMS:
- EN 20417 Provision of information by the manufacturer of medical devices;
- EN ISO 5840 Cardiovascular implants — Cardiac valve prostheses
- EN ISO 10993 series: Biological evaluation of medical devices (particularly important as a starter: Part 1: Evaluation and testing within the framework of a risk management procedure) [for the selection of necessary tests for biocompatibility and their algorithms].
- EN ISO 11135-1: Sterilization of healthcare products - Ethylene oxide - Part 1: Requirements for the development, validation and control of the application of a sterilization process for medical devices
- EN ISO 11137 series: Sterilization of health care products - Radiation
- EN ISO 11607 series: Packaging for medical devices to be sterilized in the final packaging
- EN ISO 13408 series: Aseptic manufacture of healthcare products
- EN ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes
- EN ISO 14155 Clinical investigation of medical devices for human subjects - Good clinical practice
- EN ISO 14630 Non-active surgical implants - General requirements
- EN ISO 14937 Sterilization of health care products - General requirements for the characterization of a sterilizing agent and for the development, validation and control of the application of a sterilization process for medical devices
- EN ISO 14971 Medical devices - Application of risk management on medical devices
- EN ISO 15223-1 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements
- EN ISO 15883 series: washer-disinfectors
- EN ISO 17664 Sterilization of medical devices - Information to be provided by the manufacturer for the preparation of resterilisable medical devices
- EN ISO 17665 series: Sterilization of health care products - Moist heat (steam sterilization!)
- ISO TR 20416 Medical Devices – Post market surveillance for manufacturers
- EN ISO 20417 Medical devices — Information to be supplied by the manufacturer
- EN 60601 Parts 1-Series: Medical electrical equipment -[currently 1-11] General safety requirements including essential performance characteristics
- EN 60601 Parts 2 Series: Medical Electrical Equipment - Special requirements for safety, including the essential performance features of (special species/groups of MD [currently > 50, drafts already reach up to more than 80!]
- EN IEC 62304-1 Medical Device Software - Software Life Cycle Processes
- EN 62366 Medical devices - Application of suitability for use to
- medical devices; (‘usability’)
ANNEX B - MDR: CORE LEGAL TEXTS FOR CLINICAL EVALUATION AND INVESTIGATION
ANNEX B.1: Chapter VI of MDR
CHAPTER VI CLINICAL EVALUATION AND CLINICAL INVESTIGATIONS
Article 61 Clinical evaluation
1.Confirmation of conformity with relevant general safety and performance requirements set out in Annex I under the normal conditions of the intended use of the device, and the evaluation of the undesirable side- effects and of the acceptability of the benefit-risk- ratio referred to in Sections 1 and 8 of Annex I, shall be based on clinical data providing sufficient clinical evidence, including where applicable relevant data as referred to in Annex III. The manufacturer shall specify and justify the level of clinical evidence necessary to demonstrate conformity with the relevant general safety and performance requirements. That level of clinical evidence shall be appropriate in view of the characteristics of the device and its intended purpose. To that end, manufacturers shall plan, conduct and document a clinical evaluation in accordance with this Article and Part A of Annex XIV.
2. For all class III devices and for the class IIb devices referred to in point (b) of Article 54(1), the manufacturer may, prior to its clinical evaluation and/or investigation, consult an expert panel as referred to in Article 106, with the aim of reviewing the manufacturer's intended clinical development strategy and proposals for clinical investigation. The manufacturer shall give due consideration to the views expressed by the expert panel. Such consideration shall be documented in the clinical evaluation report referred to in paragraph 12 of this Article. The manufacturer may not invoke any rights to the views expressed by the expert panel with regard to any future conformity assessment procedure.
3. A clinical evaluation shall follow a defined and methodologically sound procedure based on the following: (a) a critical evaluation of the relevant scientific literature currently available relating to the safety, performance, design characteristics and intended purpose of the device, where the following conditions are satisfied: — it is demonstrated that the device subject to clinical evaluation for the intended purpose is equivalent to the device to which the data relate, in accordance with Section 3 of Annex XIV, and — the data adequately demonstrate compliance with the relevant general safety and performance requirements; (b) a critical evaluation of the results of all available clinical investigations, taking duly into consideration whether the investigations were performed under Articles 62 to 80, any acts adopted pursuant to Article 81, and Annex XV; and (c) a consideration of currently available alternative treatment options for that purpose, if any.
4. In the case of implantable devices and class III devices, clinical investigations shall be performed, except if: — the device has been designed by modifications of a device already marketed by the same manufacturer, — the modified device has been demonstrated by the manufacturer to be equivalent to the marketed device, in accordance with Section 3 of Annex XIV and this demonstration has been endorsed by the notified body, and — the clinical evaluation of the marketed device is sufficient to demonstrate conformity of the modified device with the relevant safety and performance requirements. In this case, the notified body shall check that the PMCF plan is appropriate and includes post market studies to demonstrate the safety and performance of the device. In addition, clinical investigations need not be performed in the cases referred to in paragraph 6.
5. A manufacturer of a device demonstrated to be equivalent to an already marketed device not manufactured by him, may also rely on paragraph 4 in order not to perform a clinical investigation provided that the following conditions are fulfilled in addition to what is required in that paragraph: — the two manufacturers have a contract in place that explicitly allows the manufacturer of the second device full access to the technical documentation on an ongoing basis, and — the original clinical evaluation has been performed in compliance with the requirements of this Regulation, and the manufacturer of the second device provides clear evidence thereof to the notified body.
6. The requirement to perform clinical investigations pursuant to paragraph 4 shall not apply to implantable devices and class III devices: (a) which have been lawfully placed on the market or put into service in accordance with Directive 90/385/EEC or Directive 93/42/EEC and for which the clinical evaluation: — is based on sufficient clinical data, and — is in compliance with the relevant product-specific CS for the clinical evaluation of that kind of device, where such a CS is available; or (b) that are sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips or connectors for which the clinical evaluation is based on sufficient clinical data and is in compliance with the relevant product-specific CS, where such a CS is available.
7. Cases in which paragraph 4 is not applied by virtue of paragraph 6 shall be justified in the clinical evaluation report by the manufacturer and in the clinical evaluation assessment report by the notified body.
8.Where justified in view of well-established technologies, similar to those used in the exempted devices listed in point (b) of paragraph 6 of this Article, being used in other devices, or where justified in order to protect the health and safety of patients, users or other persons or other aspects of public health, the Commission is empowered to adopt delegated acts in accordance with Article 115 to amend the list of exempted devices referred to in the second subparagraph of Article 52(4) and in point (b) of paragraph 6 of this Article, by adding other types of implantable or class III devices to that list or removing devices therefrom.
9. In the case of the products without an intended medical purpose listed in Annex XVI, the requirement to demonstrate a clinical benefit in accordance with this Chapter and Annexes XIV and XV shall be understood as a requirement to demonstrate the performance of the device. Clinical evaluations of those products shall be based on relevant data concerning safety, including data from post-market surveillance, PMCF, and, where applicable, specific clinical investigation. Clinical investigations shall be performed for those products unless reliance on existing clinical data from an analogous medical device is duly justified.
10. Without prejudice to paragraph 4, where the demonstration of conformity with general safety and performance requirements based on clinical data is not deemed appropriate, adequate justification for any such exception shall be given based on the results of the manufacturer's risk management and on consideration of the specifics of the interaction between the device and the human body, the clinical performance intended and the claims of the manufacturer. In such a case, the manufacturer shall duly substantiate in the technical documentation referred to in Annex II why it considers a demonstration of conformity with general safety and performance requirements that is based on the results of non-clinical testing methods alone, including performance evaluation, bench testing and pre- clinical evaluation, to be adequate.
11. The clinical evaluation and its documentation shall be updated throughout the life cycle of the device concerned with clinical data obtained from the implementation of the manufacturer's PMCF plan in accordance with Part B of Annex XIV and the post-market surveillance plan referred to in Article 84. For class III devices and implantable devices, the PMCF evaluation report and, if indicated, the summary of safety and clinical performance referred to in Article 32 shall be updated at least annually with such data.
12. The clinical evaluation, its results and the clinical evidence derived from it shall be documented in a clinical evaluation report as referred to in Section 4 of Annex XIV, which, except for custom-made devices, shall be part of the technical documentation referred to in Annex II relating to the device concerned.
13.Where necessary to ensure the uniform application of Annex XIV, the Commission may, having due regard to technical and scientific progress, adopt implementing acts to the extent necessary to resolve issues of divergent interpretation and of practical application. Those implementing acts shall be adopted in accordance with the examination procedure referred to in Article 114(3).
Article 62 General requirements regarding clinical investigations conducted to demonstrate conformity of devices
1.Clinical investigations shall be designed, authorised, conducted, recorded and reported in accordance with the provisions of this Article and of Articles 63 to 80, the acts adopted pursuant to Article 81, and Annex XV, where carried out as part of the clinical evaluation for conformity assessment purposes, for one or more of the following purposes: (a) to establish and verify that, under normal conditions of use, a device is designed, manufactured and packaged in such a way that it is suitable for one or more of the specific purposes listed in point (1) of Article 2, and achieves the performance intended as specified by its manufacturer; (b) to establish and verify the clinical benefits of a device as specified by its manufacturer; (c) to establish and verify the clinical safety of the device and to determine any undesirable side-effects, under normal conditions of use of the device, and assess whether they constitute acceptable risks when weighed against the benefits to be achieved by the device.
2.Where the sponsor of a clinical investigation is not established in the Union, that sponsor shall ensure that a natural or legal person is established in the Union as its legal representative. Such legal representative shall be responsible for ensuring compliance with the sponsor's obligations pursuant to this Regulation, and shall be the addressee for all communications with the sponsor provided for in this Regulation. Any communication with that legal representative shall be deemed to be a communication with the sponsor. Member States may choose not to apply the first subparagraph to clinical investigations to be conducted solely on their territory, or on their territory and the territory of a third country, provided that they ensure that the sponsor establishes at least a contact person on their territory in respect of that clinical investigation who shall be the addressee for all communications with the sponsor provided for in this Regulation.
3.Clinical investigations shall be designed and conducted in such a way that the rights, safety, dignity and well-being of the subjects participating in a clinical investigation are protected and prevail over all other interests and the clinical data generated are scientifically valid, reliable and robust. Clinical investigations shall be subject to scientific and ethical review. The ethical review shall be performed by an ethics committee in accordance with national law. Member States shall ensure that the procedures for review by ethics committees are compatible with the procedures set out in this Regulation for the assessment of the application for authorisation of a clinical investigation. At least one lay person shall participate in the ethical review.
4. A clinical investigation as referred to in paragraph 1 may be conducted only where all of the following conditions are met: (a) the clinical investigation is the subject of an authorisation by the Member State(s) in which the clinical investigation is to be conducted, in accordance with this Regulation, unless otherwise stated; (b) an ethics committee, set up in accordance with national law, has not issued a negative opinion in relation to the clinical investigation, which is valid for that entire Member State under its national law; (c) the sponsor, or its legal representative or a contact person pursuant to paragraph 2, is established in the Union; (d) vulnerable populations and subjects are appropriately protected in accordance with Articles 64 to 68; (e) the anticipated benefits to the subjects or to public health justify the foreseeable risks and inconveniences and compliance with this condition is constantly monitored; (f) the subject or, where the subject is not able to give informed consent, his or her legally designated representative has given informed consent in accordance with Article 63; (g) the subject or, where the subject is not able to give informed consent, his or her legally designated representative, has been provided with the contact details of an entity where further information can be received in case of need; (h) the rights of the subject to physical and mental integrity, to privacy and to the protection of the data concerning him or her in accordance with Directive 95/46/EC are safeguarded; (i) the clinical investigation has been designed to involve as little pain, discomfort, fear and any other foreseeable risk as possible for the subjects, and both the risk threshold and the degree of distress are specifically defined in the clinical investigation plan and constantly monitored; (j) the medical care provided to the subjects is the responsibility of an appropriately qualified medical doctor or, where appropriate, a qualified dental practitioner or any other person entitled by national law to provide the relevant patient care under clinical investigation conditions; (k) no undue influence, including that of a financial nature, is exerted on the subject, or, where applicable, on his or her legally designated representatives, to participate in the clinical investigation; (l) the investigational device(s) in question conform(s) to the applicable general safety and performance requirements set out in Annex I apart from the aspects covered by the clinical investigation and that, with regard to those aspects, every precaution has been taken to protect the health and safety of the subjects. This includes, where appropriate, technical and biological safety testing and pre-clinical evaluation, as well as provisions in the field of occupational safety and accident prevention, taking into consideration the state of the art; (m) the requirements of Annex XV are fulfilled.
5. Any subject, or, where the subject is not able to give informed consent, his or her legally designated representative, may, without any resulting detriment and without having to provide any justification, withdraw from the clinical investigation at any time by revoking his or her informed consent. Without prejudice to Directive 95/46/EC, the withdrawal of the informed consent shall not affect the activities already carried out and the use of data obtained based on informed consent before its withdrawal.
6.The investigator shall be a person exercising a profession which is recognised in the Member State concerned as qualifying for the role of investigator on account of having the necessary scientific knowledge and experience in patient care. Other personnel involved in conducting a clinical investigation shall be suitably qualified, by education, training or experience in the relevant medical field and in clinical research methodology, to perform their tasks.
7.The facilities where the clinical investigation is to be conducted shall be suitable for the clinical investigation and shall be similar to the facilities where the device is intended to be used.
Article 63 Informed consent
1.Informed consent shall be written, dated and signed by the person performing the interview referred to in point (c) of paragraph 2, and by the subject or, where the subject is not able to give informed consent, his or her legally designated representative after having been duly informed in accordance with paragraph 2. Where the subject is unable to write, consent may be given and recorded through appropriate alternative means in the presence of at least one impartial witness. In that case, the witness shall sign and date the informed consent document. The subject or, where the subject is not able to give informed consent, his or her legally designated representative shall be provided with a copy of the document or the record, as appropriate, by which informed consent has been given. The informed consent shall be documented. Adequate time shall be given for the subject or his or her legally designated representative to consider his or her decision to participate in the clinical investigation.
2.Information given to the subject or, where the subject is not able to give informed consent, his or her legally designated representative for the purposes of obtaining his or her informed consent shall: (a) enable the subject or his or her legally designated representative to understand: (i) the nature, objectives, benefits, implications, risks and inconveniences of the clinical investigations; (ii) the subject's rights and guarantees regarding his or her protection, in particular his or her right to refuse to participate in and the right to withdraw from the clinical investigation at any time without any resulting detriment and without having to provide any justification; (iii) the conditions under which the clinical investigations is to be conducted, including the expected duration of the subject's participation in the clinical investigation; and (iv) the possible treatment alternatives, including the follow-up measures if the participation of the subject in the clinical investigation is discontinued; (b) be kept comprehensive, concise, clear, relevant, and understandable to the subject or his or her legally designated representative; (c) be provided in a prior interview with a member of the investigating team who is appropriately qualified under national law; (d) include information about the applicable damage compensation system referred to in Article 69; and (e) include the Union-wide unique single identification number of the clinical investigation referred to in Article 70(1) and information about the availability of the clinical investigation results in accordance with paragraph 6 of this Article.
3.The information referred to in paragraph 2 shall be prepared in writing and be available to the subject or, where the subject is not able to give informed consent, his or her legally designated representative.
4. In the interview referred to in point (c) of paragraph 2, special attention shall be paid to the information needs of specific patient populations and of individual subjects, as well as to the methods used to give the information.
5. In the interview referred to in point (c) of paragraph 2, it shall be verified that the subject has understood the information. 6.The subject shall be informed that a clinical investigation report and a summary presented in terms understandable to the intended user will be made available pursuant to Article 77(5) in the electronic system on clinical investigations referred to in Article 73 irrespective of the outcome of the clinical investigation, and shall be informed, to the extent possible, when they have become available.
7.This Regulation is without prejudice to national law requiring that, in addition to the informed consent given by the legally designated representative, a minor who is capable of forming an opinion and assessing the information given to him or her, shall also assent in order to participate in a clinical investigation.
Article 64 Clinical investigations on incapacitated subjects
1.In the case of incapacitated subjects who have not given, or have not refused to give, informed consent before the onset of their incapacity, a clinical investigation may be conducted only where, in addition to the conditions set out in Article 62(4), all of the following conditions are met: (a) the informed consent of their legally designated representative has been obtained; (b) the incapacitated subjects have received the information referred to in Article 63(2) in a way that is adequate in view of their capacity to understand it; (c) the explicit wish of an incapacitated subject who is capable of forming an opinion and assessing the information referred to in Article 63(2) to refuse participation in, or to withdraw from, the clinical investigation at any time, is respected by the investigator; (d) no incentives or financial inducements are given to subjects or their legally designated representatives, except for compensation for expenses and loss of earnings directly related to the participation in the clinical investigation; (e) the clinical investigation is essential with respect to incapacitated subjects and data of comparable validity cannot be obtained in clinical investigations on persons able to give informed consent, or by other research methods; (f) the clinical investigation relates directly to a medical condition from which the subject suffers; (g) there are scientific grounds for expecting that participation in the clinical investigation will produce a direct benefit to the incapacitated subject outweighing the risks and burdens involved.
2.The subject shall as far as possible take part in the informed consent procedure.
Article 65 Clinical investigations on minors
A clinical investigation on minors may be conducted only where, in addition to the conditions set out in Article 62(4), all of the following conditions are met: (a) the informed consent of their legally designated representative has been obtained; (b) the minors have received the information referred to in Article 63(2) in a way adapted to their age and mental maturity and from investigators or members of the investigating team who are trained or experienced in working with children; (c) the explicit wish of a minor who is capable of forming an opinion and assessing the information referred to in Article 63(2) to refuse participation in, or to withdraw from, the clinical investigation at any time, is respected by the investigator; (d) no incentives or financial inducements are given to the subject or his or her legally designated representative except for compensation for expenses and loss of earnings directly related to the participation in the clinical investigation; (e) the clinical investigation is intended to investigate treatments for a medical condition that only occurs in minors or the clinical investigation is essential with respect to minors to validate data obtained in clinical investigations on persons able to give informed consent or by other research methods; (f) the clinical investigation either relates directly to a medical condition from which the minor concerned suffers or is of such a nature that it can only be carried out on minors; (g) there are scientific grounds for expecting that participation in the clinical investigation will produce a direct benefit to the minor subject outweighing the risks and burdens involved; (h) the minor shall take part in the informed consent procedure in a way adapted to his or her age and mental maturity; (i) if during a clinical investigation the minor reaches the age of legal competence to give informed consent as defined in national law, his or her express informed consent shall be obtained before that subject can continue to participate in the clinical investigation.
Article 66 Clinical investigations on pregnant or breastfeeding women
A clinical investigation on pregnant or breastfeeding women may be conducted only where, in addition to the conditions set out in Article 62(4), all of the following conditions are met: (a) the clinical investigation has the potential to produce a direct benefit for the pregnant or breastfeeding woman concerned, or her embryo, foetus or child after birth, outweighing the risks and burdens involved; (b) where research is undertaken on breastfeeding women, particular care is taken to avoid any adverse impact on the health of the child; (c) no incentives or financial inducements are given to the subject except for compensation for expenses and loss of earnings directly related to the participation in the clinical investigation.
Article 67 Additional national measures
Member States may maintain additional measures regarding persons performing mandatory military service, persons deprived of liberty, persons who, due to a judicial decision, cannot take part in clinical investigations, or persons in residential care institutions.
Article 68 Clinical investigations in emergency situations
1.By way of derogation from point (f) of Article 62(4), from points (a) and (b) of Article 64(1) and from points (a) and (b) of Article 65, informed consent to participate in a clinical investigation may be obtained, and information on the clinical investigation may be given, after the decision to include the subject in the clinical investigation, provided that that decision is taken at the time of the first intervention on the subject, in accordance with the clinical investigation plan for that clinical investigation and that all of the following conditions are fulfilled: (a) due to the urgency of the situation, caused by a sudden life-threatening or other sudden serious medical condition, the subject is unable to provide prior informed consent and to receive prior information on the clinical investigation; (b) there are scientific grounds to expect that participation of the subject in the clinical investigation will have the potential to produce a direct clinically relevant benefit for the subject resulting in a measurable health-related improvement alleviating the suffering and/or improving the health of the subject, or in the diagnosis of its condition; (c) it is not possible within the therapeutic window to supply all prior information to and obtain prior informed consent from his or her legally designated representative; (d) the investigator certifies that he or she is not aware of any objections to participate in the clinical investigation previously expressed by the subject; (e) the clinical investigation relates directly to the subject's medical condition because of which it is not possible within the therapeutic window to obtain prior informed consent from the subject or from his or her legally designated representative and to supply prior information, and the clinical investigation is of such a nature that it may be conducted exclusively in emergency situations; (f) the clinical investigation poses a minimal risk to, and imposes a minimal burden on, the subject in comparison with the standard treatment of the subject's condition.
2.Following an intervention pursuant to paragraph 1 of this Article, informed consent in accordance with Article 63 shall be sought to continue the participation of the subject in the clinical investigation, and information on the clinical investigation shall be given, in accordance with the following requirements: (a) regarding incapacitated subjects and minors, the informed consent shall be sought by the investigator from his or her legally designated representative without undue delay and the information referred to in Article 63(2) shall be given as soon as possible to the subject and to his or her legally designated representative; (b) regarding other subjects, the informed consent shall be sought by the investigator without undue delay from the subject or his or her legally designated representative, whichever can be done sooner, and the information referred to in Article 63(2) shall be given as soon as possible to the subject or his or her legally designated representative, as applicable. For the purposes of point (b) where informed consent has been obtained from the legally designated representative, informed consent to continue the participation in the clinical investigation shall be obtained from the subject as soon as he or she is capable of giving informed consent. 3. If the subject or, where applicable, his or her legally designated representative does not give consent, he or she shall be informed of the right to object to the use of data obtained from the clinical investigation.
Article 69 Damage compensation
1.Member States shall ensure that systems for compensation for any damage suffered by a subject resulting from participation in a clinical investigation conducted on their territory are in place in the form of insurance, a guarantee, or a similar arrangement that is equivalent as regards its purpose and which is appropriate to the nature and the extent of the risk.
2.The sponsor and the investigator shall make use of the system referred to in paragraph 1 in the form appropriate for the Member State in which the clinical investigation is conducted.
Article 70 Application for clinical investigations
1.The sponsor of a clinical investigation shall submit an application to the Member State(s) in which the clinical investigation is to be conducted (referred to for the purposes of this Article as ‘Member State concerned’) accompanied by the documentation referred to in Chapter II of Annex XV. The application shall be submitted by means of the electronic system referred to in Article 73, which shall generate a Union wide unique single identification number for the clinical investigation, which shall be used for all relevant communication in relation to that clinical investigation. Within 10 days of it receiving the application, the Member State concerned shall notify the sponsor as to whether the clinical investigation falls within the scope of this Regulation and as to whether the application dossier is complete in accordance with Chapter II of Annex XV.
2. Within one week of any change occurring in relation to the documentation referred to in Chapter II of Annex XV, the sponsor shall update the relevant data in the electronic system referred to in Article 73 and make that change to the documentation clearly identifiable. The Member State concerned shall be notified of the update by means of that electronic system.
3. Where the Member State concerned finds that the clinical investigation applied for does not fall within the scope of this Regulation or that the application dossier is not complete, it shall inform the sponsor thereof and shall set a time limit of maximum 10 days for the sponsor to comment or to complete the application by means of the electronic system referred to in Article 73. The Member State concerned may extend this period by a maximum of 20 days where appropriate. Where the sponsor has not provided comments nor completed the application within the time limit referred to in the first subparagraph, the application shall be deemed to have lapsed. Where the sponsor considers the application does fall under the scope of this Regulation and/or is complete but the Member State concerned does not, the application shall be considered to have been rejected. The Member State concerned shall provide for an appeal procedure in respect of such refusal. The Member State concerned shall notify the sponsor within five days of receipt of the comments or of the requested additional information, whether the clinical investigation is considered as falling within the scope of this Regulation and the application is complete.
4. The Member State concerned may also extend the period referred to in paragraph 1 and 3 each by a further five days.
5. For the purposes of this Chapter, the date on which the sponsor is notified in accordance with paragraph 1 or 3 shall be the validation date of the application. Where the sponsor is not notified, the validation date shall be the last day of the periods referred to in paragraphs 1, 3 and 4 respectively.
6. During the period when the application is being assessed, the Member State may request additional information from the sponsor. The expiry of the period laid down in point (b) of paragraph 7 shall be suspended from the date of the first request until such time as the additional information has been received.
7. The sponsor may start the clinical investigation in the following circumstances: (a) in the case of investigational class I devices or in the case of non-invasive class IIa and class IIb devices, unless otherwise stated by national law, immediately after the validation date of the application pursuant to paragraph 5, and provided that a negative opinion which is valid for the entire Member State, under national law, has not been issued by an ethics committee in the Member State concerned in respect of the clinical investigation; (b) in the case of investigational devices, other than those referred to in point (a), as soon as the Member State concerned has notified the sponsor of its authorisation, and provided that a negative opinion which is valid for the entire Member State, under national law, has not been issued by an ethics committee in the Member State concerned in respect of the clinical investigation. The Member State shall notify the sponsor of the authorisation within 45 days of the validation date referred to in paragraph 5. The Member State may extend this period by a further 20 days for the purpose of consulting with experts.
8. The Commission is empowered to adopt delegated acts in accordance with Article 115 amending, in the light of technical progress and global regulatory developments, the requirements laid down in Chapter II of Annex XV.
9. In order to ensure the uniform application of the requirements laid down in Chapter II of Annex XV, the Commission may adopt implementing acts to the extent necessary to resolve issues of divergent interpretation and of practical application. Those implementing acts shall be adopted in accordance with the examination procedure referred to in Article 114(3).
Article 71 Assessment by Member States
1.Member States shall ensure that the persons validating and assessing the application, or deciding on it, do not have conflicts of interest, are independent of the sponsor, the investigators involved and of natural or legal persons financing the clinical investigation, as well as free of any other undue influence.
2. Member States shall ensure that the assessment is done jointly by an appropriate number of persons who collectively have the necessary qualifications and experience.
3. Member States shall assess whether the clinical investigation is designed in such a way that potential remaining risks to subjects or third persons, after risk minimization, are justified, when weighed against the clinical benefits to be expected. They shall, while taking into account applicable CS or harmonised standards, examine in particular: (a) the demonstration of compliance of the investigational device(s) with the applicable general safety and performance requirements, apart from the aspects covered by the clinical investigation, and whether, with regard to those aspects, every precaution has been taken to protect the health and safety of the subjects. This includes, where appropriate, assurance of technical and biological safety testing and pre clinical evaluation; (b) whether the risk-minimisation solutions employed by the sponsor are described in harmonised standards and, in those cases where the sponsor does not use harmonised standards, whether the risk-minimisation solutions provide a level of protection that is equivalent to that provided by harmonised standards; (c) whether the measures planned for the safe installation, putting into service and maintenance of the investigational device are adequate; (d) the reliability and robustness of the data generated in the clinical investigation, taking account of statistical approaches, design of the investigation and methodological aspects, including sample size, comparator and endpoints; (e) whether the requirements of Annex XV are met; (f) in the case of devices for sterile use, evidence of the validation of the manufacturer's sterilisation procedures or information on the reconditioning and sterilisation procedures which have to be conducted by the investigation site; (g) the demonstration of the safety, quality and usefulness of any components of animal or human origin or of substances, which may be considered medicinal products in accordance with Directive 2001/83/EC.
4.Member States shall refuse the authorisation of the clinical investigation if: (a) the application dossier submitted pursuant to Article 70(1) remains incomplete; (b) the device or the submitted documents, especially the investigation plan and the investigator's brochure, do not correspond to the state of scientific knowledge, and the clinical investigation, in particular, is not suitable for providing evidence for the safety, performance characteristics or benefit of the device on subjects or patients, (c) the requirements of Article 62 are not met, or (d) any assessment under paragraph 3 is negative. Member States shall provide for an appeal procedure in respect of a refusal pursuant to the first subparagraph.
Article 72 Conduct of a clinical investigation
1. The sponsor and the investigator shall ensure that the clinical investigation is conducted in accordance with the approved clinical investigation plan.
2. In order to verify that the rights, safety and well-being of subjects are protected, that the reported data are reliable and robust, and that the conduct of the clinical investigation is in compliance with the requirements of this Regulation, the sponsor shall ensure adequate monitoring of the conduct of a clinical investigation. The extent and nature of the monitoring shall be determined by the sponsor on the basis of an assessment that takes into consideration all characteristics of the clinical investigation including the following: (a) the objective and methodology of the clinical investigation; and (b) the degree of deviation of the intervention from normal clinical practice.
3. All clinical investigation information shall be recorded, processed, handled, and stored by the sponsor or investigator, as applicable, in such a way that it can be accurately reported, interpreted and verified while the confidentiality of records and the personal data of the subjects remain protected in accordance with the applicable law on personal data protection.
4. Appropriate technical and organisational measures shall be implemented to protect information and personal data processed against unauthorised or unlawful access, disclosure, dissemination, alteration, or destruction or accidental loss, in particular where the processing involves transmission over a network.
5. Member States shall inspect, at an appropriate level, investigation site(s) to check that clinical investigations are conducted in accordance with the requirements of this Regulation and with the approved investigation plan.
6. The sponsor shall establish a procedure for emergency situations which enables the immediate identification and, where necessary, an immediate recall of the devices used in the investigation.
Article 73 Electronic system on clinical investigations
1. The Commission shall, in collaboration with the Member States, set up, manage and maintain an electronic system: (a) to create the single identification numbers for clinical investigations referred to in Article 70(1); (b) to be used as an entry point for the submission of all applications or notifications for clinical investigations referred to in Articles 70, 74, 75 and 78 and for all other submission of data, or processing of data in this context; (c) for the exchange of information relating to clinical investigations in accordance with this Regulation between the Member States and between them and the Commission including the exchange of information referred to in Articles 70 and 76; (d) for information to be provided by the sponsor in accordance with Article 77, including the clinical investigation report and its summary as required in paragraph 5 of that Article; (e) for reporting on serious adverse events and device deficiencies and related updates referred to in Article 80.
2.When setting up the electronic system referred in paragraph 1 of this Article, the Commission shall ensure that it is interoperable with the EU database for clinical trials on medicinal products for human use set up in accordance with Article 81 of Regulation (EU) No 536/2014 of the European Parliament and of the Council(1) as concerns combined clinical investigations of devices with a clinical trial under that Regulation.
3.The information referred to in point (c) of paragraph 1 shall only be accessible to the Member States and the Commission. The information referred to in the other points of that paragraph shall be accessible to the public, unless, for all or parts of that information, confidentiality of the information is justified on any of the following grounds: (a) protection of personal data in accordance with Regulation (EC) No 45/2001; (b) protection of commercially confidential information, especially in the investigators brochure, in particular through taking into account the status of the conformity assessment for the device, unless there is an overriding public interest in disclosure; (c) effective supervision of the conduct of the clinical investigation by the Member State(s) concerned.
4. No personal data of subjects shall be publicly available.
5. The user interface of the electronic system referred to in paragraph 1 shall be available in all official languages of the Union.
Article 74 Clinical investigations regarding devices bearing the CE marking
1.Where a clinical investigation is to be conducted to further assess, within the scope of its intended purpose, a device which already bears the CE marking in accordance with Article 20(1), (‘PMCF investigation’), and where the investigation would involve submitting subjects to procedures additional to those performed under the normal conditions of use of the device and those additional procedures are invasive or burdensome, the sponsor shall notify the Member States concerned at least 30 days prior to its commencement by means of the electronic system referred to in Article 73. The sponsor shall include the documentation referred to in Chapter II of Annex XV as part of the notification. Points (b) to (k) and (m) of Article 62(4), Article 75, Article 76, Article 77, Article 80(5) and the relevant provisions of Annex XV shall apply to PMCF investigations.
2. Where a clinical investigation is to be conducted to assess, outside the scope of its intended purpose, a device which already bears the CE marking in accordance with Article 20(1), Articles 62 to 81 shall apply.
Article 75 Substantial modifications to clinical investigations
1. If a sponsor intends to introduce modifications to a clinical investigation that are likely to have a substantial impact on the safety, health or rights of the subjects or on the robustness or reliability of the clinical data generated by the investigation, it shall notify, within one week, by means of the electronic system referred to in Article 73 the Member State(s) in which the clinical investigation is being or is to be conducted of the reasons for and the nature of those modifications. The sponsor shall include an updated version of the relevant documentation referred to in Chapter II of Annex XV as part of the notification. Changes to the relevant documentation shall be clearly identifiable.
2. The Member State shall assess any substantial modification to the clinical investigation in accordance with the procedure laid down in Article 71.
3. The sponsor may implement the modifications referred to in paragraph 1 at the earliest 38 days after the notification referred to in that paragraph, unless: (a) the Member State in which the clinical investigation is being or is to be conducted has notified the sponsor of its refusal based on the grounds referred to in Article 71(4) or on considerations of public health, subject and user safety or health, of public policy, or (b) an ethics committee in that Member State has issued a negative opinion in relation to the substantial modification to the clinical investigation, which, in accordance with national law, is valid for that entire Member State.
4. The Member State(s) concerned may extend the period referred to in paragraph 3 by a further seven days, for the purpose of consulting with experts.
Article 76 Corrective measures to be taken by Member States and information exchange between Member States
1. Where a Member State in which a clinical investigation is being or is to be conducted has grounds for considering that the requirements set out in this Regulation are not met, it may take at least any of the following measures on its territory: (a) revoke the authorisation for the clinical investigation; (b) suspend or terminate the clinical investigation; (c) require the sponsor to modify any aspect of the clinical investigation.
2. Before the Member State concerned takes any of the measures referred to in paragraph 1 it shall, except where immediate action is required, ask the sponsor or the investigator or both for their opinion. That opinion shall be delivered within seven days
3. Where a Member State has taken a measure referred to in paragraph 1 of this Article or has refused a clinical investigation, or has been notified by the sponsor of the early termination of a clinical investigation on safety grounds, that Member State shall communicate the corresponding decision and the grounds therefor to all Member States and the Commission by means of the electronic system referred to in Article 73.
4. Where an application is withdrawn by the sponsor prior to a decision by a Member State, that information shall be made available through the electronic system referred to in Article 73 to all Member States and the Commission.
Article 77 Information from the sponsor at the end of a clinical investigation or in the event of a temporary halt or early termination
1. If the sponsor has temporarily halted a clinical investigation or has terminated a clinical investigation early, it shall inform within 15 days the Member State in which that clinical investigation has been temporarily halted or terminated early, through the electronic system referred to in Article 73, of the temporary halt or early termination, providing a justification. In the event that the sponsor has temporarily halted or terminated early the clinical investigation on safety grounds, it shall inform all Member States in which that clinical investigation is being conducted thereof within 24 hours.
2. The end of a clinical investigation shall be deemed to coincide with the last visit of the last subject unless another point in time for such end is set out in the clinical investigation plan.
3. The sponsor shall notify each Member State in which a clinical investigation was being conducted of the end of that clinical investigation in that Member State. That notification shall be made within 15 days of the end of the clinical investigation in relation to that Member State.
4. If an investigation is conducted in more than one Member State, the sponsor shall notify all Member States in which that clinical investigation was conducted of the end of the clinical investigation in all Member States. That notification shall be made within 15 days of that end of the clinical investigation.
5. Irrespective of the outcome of the clinical investigation, within one year of the end of the clinical investigation or within three months of the early termination or temporary halt, the sponsor shall submit to the Member States in which a clinical investigation was conducted a clinical investigation report as referred to in Section 2.8 of Chapter I and Section 7 of Chapter III of Annex XV. The clinical investigation report shall be accompanied by a summary presented in terms that are easily understandable to the intended user. Both the report and summary shall be submitted by the sponsor by means of the electronic system referred to in Article 73. Where, for scientific reasons, it is not possible to submit the clinical investigation report within one year of the end of the investigation, it shall be submitted as soon as it is available. In such case, the clinical investigation plan referred to in Section 3 of Chapter II of Annex XV shall specify when the results of the clinical investigation are going to be available, together with a justification.
6. The Commission shall issue guidelines regarding the content and structure of the summary of the clinical investigation report. In addition, the Commission may issue guidelines for the formatting and sharing of raw data, for cases where the sponsor decides to share raw data on a voluntary basis. Those guidelines may take as a basis and adapt, where possible, existing guidelines for sharing of raw data in the field of clinical investigations.
7. The summary and the clinical investigation report referred to in paragraph 5 of this Article shall become publicly accessible through the electronic system referred to in Article 73, at the latest when the device is registered in accordance with Article 29 and before it is placed on the market. In cases of early termination or temporary halt, the summary and the report shall become publicly accessible immediately after submission. If the device is not registered in accordance with Article 29 within one year of the summary and the report having been entered into the electronic system pursuant to paragraph 5 of this Article, they shall become publicly accessible at that point in time.
Article 78 Coordinated assessment procedure for clinical investigations
1. By means of the electronic system referred to in Article 73, the sponsor of a clinical investigation to be conducted in more than one Member State may submit, for the purpose of Article 70, a single application that, upon receipt, is transmitted electronically to all Member States in which the clinical investigation is to be conducted.
2. The sponsor shall propose in the single application referred to in paragraph 1 that one of the Member States in which the clinical investigation is to be conducted acts as coordinating Member State. The Member States in which the clinical investigation is to be conducted shall, within six days of submission of the application, agree on one of them taking the role of the coordinating Member State. If they do not agree on a coordinating Member State, the coordinating Member State proposed by the sponsor shall assume that role.
3. Under the direction of the coordinating Member State referred to in paragraph 2, the Member States concerned shall coordinate their assessment of the application, in particular of the documentation referred to in Chapter II of Annex XV. However, the completeness of the documentation referred to in Sections 1.13, 3.1.3, 4.2, 4.3 and 4.4 of Chapter II of Annex XV shall be assessed separately by each Member State concerned in accordance with Article 70(1) to (5).
4. With regard to documentation other than that referred to in the second subparagraph of paragraph 3, the coordinating Member State shall: (a) within six days of receipt of the single application, notify the sponsor that it is the coordinating Member State (‘notification date’); (b) for the purpose of the validation of the application, take into account any considerations submitted within seven days of the notification date by any Member State concerned; (c) within 10 days of the notification date, assess whether the clinical investigation falls within the scope of this Regulation and whether the application is complete, and shall notify the sponsor accordingly. Article 70(1) and (3) to (5) shall apply to the coordinating Member State in relation to that assessment; (d) establish the results of its assessment in a draft assessment report to be transmitted within 26 days of the validation date to the Member States concerned. By day 38 after the validation date, the other Member States concerned shall transmit their comments and proposals on the draft assessment report and the underlying application to the coordinating Member State which shall take due account of those comments and proposals in its finalisation of the final assessment report, to be transmitted within 45 days of the validation date to the sponsor and the other Member States concerned. The final assessment report shall be taken into account by all Member States concerned when deciding on the sponsor's application in accordance with Article 70(7).
5. As regards the assessment of the documentation referred to in the second subparagraph of paragraph 3, each Member State concerned may request, on a single occasion, additional information from the sponsor. The sponsor shall submit the requested additional information within the period set by the Member State concerned, which shall not exceed 12 days from the receipt of the request. The expiry of the last deadline pursuant to point (d) of paragraph 4 shall be suspended from the date of the request until such time as the additional information has been received.
6. For class IIb and class III devices, the coordinating Member State may also extend the periods referred to in paragraph 4 by a further 50 days, for the purpose of consulting with experts.
7. The Commission may, by means of implementing acts, further specify the procedures and timescales for coordinated assessments to be taken into account by Member States concerned when deciding on the sponsor's application. Such implementing acts may also set out the procedures and timescales for coordinated assessment in the case of substantial modifications pursuant to paragraph 12 of this Article, in the case of reporting of adverse events pursuant to Article 80(4) and in the case of clinical investigations of combination products between medical devices and medicinal products, where the latter are under a concurrent coordinated assessment of a clinical trial under Regulation (EU) No 536/2014. Those implementing acts shall be adopted in accordance with the examination procedure referred to in Article 114(3).
8. Where the conclusion of the coordinating Member State concerning the area of coordinated assessment is that the conduct of the clinical investigation is acceptable or acceptable subject to compliance with specific conditions, that conclusion shall be deemed to be the conclusion of all Member States concerned. Notwithstanding the first subparagraph, a Member State concerned may only disagree with the conclusion of the coordinating Member State concerning the area of coordinated assessment on the following grounds: (a) when it considers that participation in the clinical investigation would lead to a subject receiving treatment inferior to that received in normal clinical practice in that Member State concerned; (b) infringement of national law; or (c) considerations as regards subject safety and data reliability and robustness submitted under point (b) of paragraph 4. Where one of the Member States concerned disagrees with the conclusion on the basis of the second subparagraph of this paragraph, it shall communicate its disagreement, together with a detailed justification, through the electronic system referred to in Article 73, to the Commission, to all other Member States concerned and to the sponsor.
9. Where the conclusion of the coordinating Member State concerning the area of coordinated assessment is that the clinical investigation is not acceptable, that conclusion shall be deemed to be the conclusion of all Member States concerned.
10. A Member State concerned shall refuse to authorise a clinical investigation if it disagrees with the conclusion of the coordinating Member State as regards any of the grounds referred to in the second subparagraph of paragraph 8, or if it finds, on duly justified grounds, that the aspects addressed in Sections 1.13, 3.1.3, 4.2, 4.3 and 4.4 of Chapter II of Annex XV are not complied with, or where an ethics committee has issued a negative opinion in relation to that clinical investigation, which is valid, in accordance with national law, for that entire Member State. That Member State shall provide for an appeal procedure in respect of such refusal.
11. Each Member State concerned shall notify the sponsor through the electronic system referred to in Article 73 as to whether the clinical investigation is authorised, whether it is authorised subject to conditions, or whether authorisation has been refused. Notification shall be done by way of one single decision within five days of the transmission, pursuant to point (d) of paragraph 4, by the coordinating Member State of the final assessment report. Where an authorisation of a clinical investigation is subject to conditions, those conditions may only be such that, by their nature, they cannot be fulfilled at the time of that authorisation.
12. Any substantial modifications as referred to in Article 75 shall be notified to the Member States concerned by means of the electronic system referred to in Article 73. Any assessment as to whether there are grounds for disagreement as referred to in the second subparagraph of paragraph 8 of this Article shall be carried out under the direction of the coordinating Member State, except for substantial modifications concerning Sections 1.13, 3.1.3, 4.2, 4.3 and 4.4 of Chapter II of Annex XV, which shall be assessed separately by each Member State concerned.
13. The Commission shall provide administrative support to the coordinating Member State in the accomplishment of its tasks under this Chapter.
14. The procedure set out in this Article shall, until 27 May 2027, be applied only by those of the Member States in which the clinical investigation is to be conducted which have agreed to apply it. After 27 May 2027, all Member States shall be required to apply that procedure.
Article 79 Review of coordinated assessment procedure
By 27 May 2026, the Commission shall submit to the European Parliament and to the Council a report on experience gained from the application of Article 78 and, if necessary, propose a review of Article 78(14) and point (h) of Article 123(3).
Article 80 Recording and reporting of adverse events that occur during clinical investigations
1.The sponsor shall fully record all of the following: (a) any adverse event of a type identified in the clinical investigation plan as being critical to the evaluation of the results of that clinical investigation; (b) any serious adverse event; (c) any device deficiency that might have led to a serious adverse event if appropriate action had not been taken, intervention had not occurred, or circumstances had been less fortunate; (d) any new findings in relation to any event referred to in points (a) to (c).
2. The sponsor shall report, without delay to all Member States in which the clinical investigation is being conducted, all of the following by means of the electronic system referred to in Article 73: (a) any serious adverse event that has a causal relationship with the investigational device, the comparator or the investigation procedure or where such causal relationship is reasonably possible; (b) any device deficiency that might have led to a serious adverse event if appropriate action had not been taken, intervention had not occurred, or circumstances had been less fortunate; (c) any new findings in relation to any event referred to in points (a) and (b). The period for reporting shall take account of the severity of the event. Where necessary to ensure timely reporting, the sponsor may submit an initial report that is incomplete followed up by a complete report. Upon request by any Member State in which the clinical investigation is being conducted, the sponsor shall provide all information referred to in paragraph 1.
3. The sponsor shall also report to the Member States in which the clinical investigation is being conducted any event referred to in paragraph 2 of this Article that occurred in third countries in which a clinical investigation is performed under the same clinical investigation plan as the one applying to a clinical investigation covered by this Regulation by means of the electronic system referred to in Article 73.
4. In the case of a clinical investigation for which the sponsor has used the single application referred to in Article 78, the sponsor shall report any event as referred to in paragraph 2 of this Article by means of the electronic system referred to in Article 73. Upon receipt, this report shall be transmitted electronically to all Member States in which the clinical investigation is being conducted. Under the direction of the coordinating Member State referred to in Article 78(2), the Member States shall coordinate their assessment of serious adverse events and device deficiencies to determine whether to modify, suspend or terminate the clinical investigation or whether to revoke the authorisation for that clinical investigation. This paragraph shall not affect the rights of the other Member States to perform their own evaluation and to adopt measures in accordance with this Regulation in order to ensure the protection of public health and patient safety. The coordinating Member State and the Commission shall be kept informed of the outcome of any such evaluation and the adoption of any such measures.
5. In the case of PMCF investigations referred to in Article 74(1), the provisions on vigilance laid down in Articles 87 to 90 and in the acts adopted pursuant to Article 91 shall apply instead of this Article.
6. Notwithstanding paragraph 5, this Article shall apply where a causal relationship between the serious adverse event and the preceding investigational procedure has been established.
Article 81 Implementing acts
The Commission may, by means of implementing acts, establish the detailed arrangements and procedural aspects necessary for the implementation of this Chapter as regards the following: (a) harmonised electronic forms for the application for clinical investigations and their assessment as referred to in Articles 70 and 78, taking into account specific categories or groups of devices; (b) the functioning of the electronic system referred to in Article 73; (c) harmonised electronic forms for the notification of PMCF investigations as referred to in Article 74(1), and of substantial modifications as referred to in Article 75; (d) the exchange of information between Member States as referred to in Article 76; (e) harmonised electronic forms for the reporting of serious adverse events and device deficiencies as referred to in Article 80; (f) the timelines for the reporting of serious adverse events and device deficiencies, taking into account the severity of the event to be reported as referred to in Article 80; (g) uniform application of the requirements regarding the clinical evidence or data needed to demonstrate compliance with the general safety and performance requirements set out in Annex I. The implementing acts referred to in the first paragraph shall be adopted in accordance with the examination procedure referred to in Article 114(3).
Article 82 Requirements regarding other clinical investigations
1. Clinical investigations, not performed pursuant to any of the purposes listed in Article 62(1), shall comply with the provisions of Article 62 (2) and (3), points (b), (c), (d), (f), (h), and (l) of Article 62(4) and Article 62(6).
2. In order to protect the rights, safety, dignity and well-being of subjects and the scientific and ethical integrity of clinical investigations not performed for any of the purposes listed in Article 62(1), each Member State shall define any additional requirements for such investigations, as appropriate for each Member State concerned.
Annex B.2: Annex XIV
ANNEX XIV CLINICAL EVALUATION AND POST-MARKET CLINICAL FOLLOW-UP
PART A CLINICAL EVALUATION
1. To plan, continuously conduct and document a clinical evaluation, manufacturers shall: (a) establish and update a clinical evaluation plan, which shall include at least: — an identification of the general safety and performance requirements that require support from relevant clinical data; — a specification of the intended purpose of the device; — a clear specification of intended target groups with clear indications and contra-indications; — a detailed description of intended clinical benefits to patients with relevant and specified clinical outcome parameters; — a specification of methods to be used for examination of qualitative and quantitative aspects of clinical safety with clear reference to the determination of residual risks and side-effects; — an indicative list and specification of parameters to be used to determine, based on the state of the art in medicine, the acceptability of the benefit-risk ratio for the various indications and for the intended purpose or purposes of the device; — an indication how benefit-risk issues relating to specific components such as use of pharmaceutical, non- viable animal or human tissues, are to be addressed; and — a clinical development plan indicating progression from exploratory investigations, such as first-in-man studies, feasibility and pilot studies, to confirmatory investigations, such as pivotal clinical investigations, and a PMCF as referred to in Part B of this Annex with an indication of milestones and a description of potential acceptance criteria; (b) identify available clinical data relevant to the device and its intended purpose and any gaps in clinical evidence through a systematic scientific literature review; (c) appraise all relevant clinical data by evaluating their suitability for establishing the safety and performance of the device; (d) generate, through properly designed clinical investigations in accordance with the clinical development plan, any new or additional clinical data necessary to address outstanding issues; and (e) analyse all relevant clinical data in order to reach conclusions about the safety and clinical performance of the device including its clinical benefits.
2. The clinical evaluation shall be thorough and objective, and take into account both favourable and unfavourable data. Its depth and extent shall be proportionate and appropriate to the nature, classification, intended purpose and risks of the device in question, as well as to the manufacturer's claims in respect of the device.
3. A clinical evaluation may be based on clinical data relating to a device for which equivalence to the device in question can be demonstrated. The following technical, biological and clinical characteristics shall be taken into consideration for the demonstration of equivalence: — Technical: the device is of similar design; is used under similar conditions of use; has similar specifications and properties including physicochemical properties such as intensity of energy, tensile strength, viscosity, surface characteristics, wavelength and software algorithms; uses similar deployment methods, where relevant; has similar principles of operation and critical performance requirements; — Biological: the device uses the same materials or substances in contact with the same human tissues or body fluids for a similar kind and duration of contact and similar release characteristics of substances, including degradation products and leachables; — Clinical: the device is used for the same clinical condition or purpose, including similar severity and stage of disease, at the same site in the body, in a similar population, including as regards age, anatomy and physiology; has the same kind of user; has similar relevant critical performance in view of the expected clinical effect for a specific intended purpose. The characteristics listed in the first paragraph shall be similar to the extent that there would be no clinically significant difference in the safety and clinical performance of the device. Considerations of equivalence shall be based on proper scientific justification. It shall be clearly demonstrated that manufacturers have sufficient levels of access to the data relating to devices with which they are claiming equivalence in order to justify their claims of equivalence.
4. The results of the clinical evaluation and the clinical evidence on which it is based shall be documented in a clinical evaluation report which shall support the assessment of the conformity of the device. The clinical evidence together with non-clinical data generated from non clinical testing methods and other relevant documentation shall allow the manufacturer to demonstrate conformity with the general safety and performance requirements and shall be part of the technical documentation for the device in question. Both favourable and unfavourable data considered in the clinical evaluation shall be included in the technical documentation.
PART B POST-MARKET CLINICAL FOLLOW-UP
5. PMCF shall be understood to be a continuous process that updates the clinical evaluation referred to in Article 61 and Part A of this Annex and shall be addressed in the manufacturer's post-market surveillance plan. When conducting PMCF, the manufacturer shall proactively collect and evaluate clinical data from the use in or on humans of a device which bears the CE marking and is placed on the market or put into service within its intended purpose as referred to in the relevant conformity assessment procedure, with the aim of confirming the safety and performance throughout the expected lifetime of the device, of ensuring the continued acceptability of identified risks and of detecting emerging risks on the basis of factual evidence.
6. PMCF shall be performed pursuant to a documented method laid down in a PMCF plan.
6.1. The PMCF plan shall specify the methods and procedures for proactively collecting and evaluating clinical data with the aim of: (a) confirming the safety and performance of the device throughout its expected lifetime, (b) identifying previously unknown side-effects and monitoring the identified side-effects and contraindications, (c) identifying and analysing emergent risks on the basis of factual evidence, (d) ensuring the continued acceptability of the benefit-risk ratio referred to in Sections 1 and 9 of Annex I, and (e) identifying possible systematic misuse or off-label use of the device, with a view to verifying that the intended purpose is correct.
6.2. The PMCF plan shall include at least: (a) the general methods and procedures of the PMCF to be applied, such as gathering of clinical experience gained, feedback from users, screening of scientific literature and of other sources of clinical data; (b) the specific methods and procedures of PMCF to be applied, such as evaluation of suitable registers or PMCF studies; (c) a rationale for the appropriateness of the methods and procedures referred to in points (a) and (b); (d) a reference to the relevant parts of the clinical evaluation report referred to in Section 4 and to the risk management referred to in Section 3 of Annex I; 5.5.2017 L 117/165 Official Journal of the European Union EN (e) the specific objectives to be addressed by the PMCF; (f) an evaluation of the clinical data relating to equivalent or similar devices; (g) reference to any relevant CS, harmonised standards when used by the manufacturer, and relevant guidance on PMCF; and (h) a detailed and adequately justified time schedule for PMCF activities (e.g. analysis of PMCF data and reporting) to be undertaken by the manufacturer.
7. The manufacturer shall analyse the findings of the PMCF and document the results in a PMCF evaluation report that shall be part of the clinical evaluation report and the technical documentation.
8. The conclusions of the PMCF evaluation report shall be taken into account for the clinical evaluation referred to in Article 61 and Part A of this Annex and in the risk management referred to in Section 3 of Annex I. If, through the PMCF, the need for preventive and/or corrective measures has been identified, the manufacturer shall implement them.
ANNEX XV CLINICAL INVESTIGATIONS
CHAPTER I GENERAL REQUIREMENTS
1. Ethical principles Each step in the clinical investigation, from the initial consideration of the need for and justification of the study to the publication of the results, shall be carried out in accordance with recognised ethical principles.
2. Methods 2.1. Clinical investigations shall be performed on the basis of an appropriate plan of investigation reflecting the latest scientific and technical knowledge and defined in such a way as to confirm or refute the manufacturer's claims regarding the safety, performance and aspects relating to benefit-risk of devices as referred to in Article 62(1); the clinical investigations shall include an adequate number of observations to guarantee the scientific validity of the conclusions. The rationale for the design and chosen statistical methodology shall be presented as further described in Section 3.6 of Chapter II of this Annex. 2.2. The procedures used to perform the clinical investigation shall be appropriate to the device under investigation. 2.3. The research methodologies used to perform the clinical investigation shall be appropriate to the device under investigation. 2.4. Clinical investigations shall be performed in accordance with the clinical investigation plan by a sufficient number of intended users and in a clinical environment that is representative of the intended normal conditions of use of the device in the target patient population. Clinical investigations shall be in line with the clinical evaluation plan as referred to in Part A of Annex XIV. 2.5. All the appropriate technical and functional features of the device, in particular those involving safety and performance, and their expected clinical outcomes shall be appropriately addressed in the investigational design. A list of the technical and functional features of the device and the related expected clinical outcomes shall be provided. 2.6. The endpoints of the clinical investigation shall address the intended purpose, clinical benefits, performance and safety of the device. The endpoints shall be determined and assessed using scientifically valid methodologies. The primary endpoint shall be appropriate to the device and clinically relevant. 2.7. Investigators shall have access to the technical and clinical data regarding the device. Personnel involved in the conduct of an investigation shall be adequately instructed and trained in the proper use of the investigational device, and as regards the clinical investigation plan and good clinical practice. This training shall be verified and where necessary arranged by the sponsor and documented appropriately. 2.8. The clinical investigation report, signed by the investigator, shall contain a critical evaluation of all the data collected during the clinical investigation, and shall include any negative findings.
CHAPTER II DOCUMENTATION REGARDING THE APPLICATION FOR CLINICAL INVESTIGATION
For investigational devices covered by Article 62, the sponsor shall draw up and submit the application in accordance with Article 70 accompanied by the following documents: 1. Application form The application form shall be duly filled in, containing information regarding: 1.1. name, address and contact details of the sponsor and, if applicable, name, address and contact details of its contact person or legal representative in accordance with Article 62(2) established in the Union; 1.2. if different from those in Section 1.1, name, address and contact details of the manufacturer of the device intended for clinical investigation and, if applicable, of its authorised representative; 1.3. title of the clinical investigation; 1.4. status of the clinical investigation application (i.e. first submission, resubmission, significant amendment); 1.5. details and/or reference to the clinical evaluation plan; 1.6. If the application is a resubmission with regard to a device for which an application has been already submitted, the date or dates and reference number or numbers of the earlier application or in the case of significant amendment, reference to the original application. The sponsor shall identify all of the changes from the previous application together with a rationale for those changes, in particular, whether any changes have been made to address conclusions of previous competent authority or ethics committee reviews; 1.7. if the application is submitted in parallel with an application for a clinical trial in accordance with Regulation (EU) No 536/2014, reference to the official registration number of the clinical trial; 1.8. identification of the Member States and third countries in which the clinical investigation is to be conducted as part of a multicentre or multinational study at the time of application; 1.9.a brief description of the investigational device, its classification and other information necessary for the identification of the device and device type; 1.10. information as to whether the device incorporates a medicinal substance, including a human blood or plasma derivative or whether it is manufactured utilising non-viable tissues or cells of human or animal origin, or their derivatives; 1.11. summary of the clinical investigation plan including the objective or objectives of the clinical investigation, the number and gender of subjects, criteria for subject selection, whether there are subjects under 18 years of age, design of the investigation such as controlled and/or randomised studies, planned dates of commencement and of completion of the clinical investigation; 1.12. if applicable, information regarding a comparator device, its classification and other information necessary for the identification of the comparator device; 1.13. evidence from the sponsor that the clinical investigator and the investigational site are capable of conducting the clinical investigation in accordance with the clinical investigation plan; 1.14. details of the anticipated start date and duration of the investigation; 1.15. details to identify the notified body, if already involved at the stage of application for a clinical investigation; 1.16. confirmation that the sponsor is aware that the competent authority may contact the ethics committee that is assessing or has assessed the application; and 1.17. the statement referred to in Section 4.1.
2. Investigator's Brochure The investigator's brochure (IB) shall contain the clinical and non-clinical information on the investigational device that is relevant for the investigation and available at the time of application. Any updates to the IB or other relevant information that is newly available shall be brought to the attention of the investigators in a timely manner. The IB shall be clearly identified and contain in particular the following information: 2.1.Identification and description of the device, including information on the intended purpose, the risk classification and applicable classification rule pursuant to Annex VIII, design and manufacturing of the device and reference to previous and similar generations of the device. 2.2. Manufacturer's instructions for installation, maintenance, maintaining hygiene standards and for use, including storage and handling requirements, as well as, to the extent that such information is available, information to be placed on the label, and instructions for use to be provided with the device when placed on the market. In addition, information relating to any relevant training required. 2.3. Pre clinical evaluation based on relevant pre-clinical testing and experimental data, in particular regarding in- design calculations, in vitro tests, ex vivo tests, animal tests, mechanical or electrical tests, reliability tests, sterilisation validation, software verification and validation, performance tests, evaluation of biocompatibility and biological safety, as applicable. 2.4. Existing clinical data, in particular: — from relevant scientific literature available relating to the safety, performance, clinical benefits to patients, design characteristics and intended purpose of the device and/or of equivalent or similar devices; — other relevant clinical data available relating to the safety, performance, clinical benefits to patients, design characteristics and intended purpose of equivalent or similar devices of the same manufacturer, including length of time on the market and a review of performance, clinical benefit and safety-related issues and any corrective actions taken. 2.5. Summary of the benefit-risk analysis and the risk management, including information regarding known or foreseeable risks, any undesirable effects, contraindications and warnings. 2.6. In the case of devices that incorporate a medicinal substance, including a human blood or plasma derivative or devices manufactured utilising non-viable tissues or cells of human or animal origin, or their derivatives, detailed information on the medicinal substance or on the tissues, cells or their derivatives, and on the compliance with the relevant general safety and performance requirements and the specific risk management in relation to the substance or tissues, cells or their derivatives, as well as evidence for the added value of incorporation of such constituents in relation to the clinical benefit and/or safety of the device. 2.7. A list detailing the fulfilment of the relevant general safety and performance requirements set out in Annex I, including the standards and CS applied, in full or in part, as well as a description of the solutions for fulfilling the relevant general safety and performance requirements, in so far as those standards and CS have not or have only been partly fulfilled or are lacking. 2.8. A detailed description of the clinical procedures and diagnostic tests used in the course of the clinical investigation and in particular information on any deviation from normal clinical practice.
3. Clinical Investigation Plan The clinical investigation plan (CIP) shall set out the rationale, objectives, design methodology, monitoring, conduct, record-keeping and the method of analysis for the clinical investigation. It shall contain in particular the information as laid down in this Annex. If part of this information is submitted in a separate document, it shall be referenced in the CIP. 3.1. General 3.1.1. Single identification number of the clinical investigation, as referred to in Article 70(1). 3.1.2. Identification of the sponsor — name, address and contact details of the sponsor and, where applicable, the name, address and contact details of the sponsor's contact person or legal representative in accordance with Article 62(2) established in the Union. 3.1.3. Information on the principal investigator at each investigational site, the coordinating investigator for the investigation, the address details for each investigational site and the emergency contact details for the principal investigator at each site. The roles, responsibilities and qualifications of the various kinds of investigators shall be specified in the CIP. 3.1.4. A brief description of how the clinical investigation is financed and a brief description of the agreement between the sponsor and the site. 3.1.5. Overall synopsis of the clinical investigation, in an official Union language determined by the Member State concerned. 3.2. Identification and description of the device, including its intended purpose, its manufacturer, its traceability, the target population, materials coming into contact with the human body, the medical or surgical procedures involved in its use and the necessary training and experience for its use, background literature review, the current state of the art in clinical care in the relevant field of application and the proposed benefits of the new device. 3.3. Risks and clinical benefits of the device to be examined, with justification of the corresponding expected clinical outcomes in the clinical investigation plan. 3.4. Description of the relevance of the clinical investigation in the context of the state of the art of clinical practice. 3.5. Objectives and hypotheses of the clinical investigation. 3.6. Design of the clinical investigation with evidence of its scientific robustness and validity. 3.6.1. General information such as type of investigation with rationale for choosing it, for its endpoints and for its variables as set out in the clinical evaluation plan. 3.6.2. Information on the investigational device, on any comparator and on any other device or medication to be used in the clinical investigation. 3.6.3. Information on subjects, selection criteria, size of investigation population, representativeness of investigation population in relation to target population and, if applicable, information on vulnerable subjects involved such as children, pregnant women, immuno compromised or, elderly subjects. 3.6.4. Details of measures to be taken to minimise bias, such as randomisation, and management of potential confounding factors. 3.6.5. Description of the clinical procedures and diagnostic methods relating to the clinical investigation and in particular highlighting any deviation from normal clinical practice. 3.6.6. Monitoring plan. 3.7. Statistical considerations, with justification, including a power calculation for the sample size, if applicable. 3.8. Data management. 3.9. Information about any amendments to the CIP. 3.10. Policy regarding follow-up and management of any deviations from the CIP at the investigational site and clear prohibition of use of waivers from the CIP. 3.11. Accountability regarding the device, in particular control of access to the device, follow-up in relation to the device used in the clinical investigation and the return of unused, expired or malfunctioning devices. 3.12. Statement of compliance with the recognised ethical principles for medical research involving humans, and the principles of good clinical practice in the field of clinical investigations of devices, as well as with the applicable regulatory requirements. 3.13. Description of the Informed consent process. 3.14. Safety reporting, including definitions of adverse events and serious adverse events, device deficiencies, procedures and timelines for reporting. 3.15. Criteria and procedures for follow-up of subjects following the end, temporary halt or early termination of an investigation, for follow-up of subjects who have withdrawn their consent and procedures for subjects lost to follow-up. Such procedures shall for implantable devices, cover as a minimum traceability. 3.16. A description of the arrangements for taking care of the subjects after their participation in the clinical investigation has ended, where such additional care is necessary because of the subjects' participation in the clinical investigation and where it differs from that normally expected for the medical condition in question. 3.17. Policy as regards the establishment of the clinical investigation report and publication of results in accordance with the legal requirements and the ethical principles referred to in Section 1 of Chapter I. 3.18.List of the technical and functional features of the device, with specific mention of those covered by the investigation. 3.19. Bibliography.
4. Other information 4.1. A signed statement by the natural or legal person responsible for the manufacture of the investigational device that the device in question conforms to the general safety and performance requirements apart from the aspects covered by the clinical investigation and that, with regard to those aspects, every precaution has been taken to protect the health and safety of the subject. 4.2. Where applicable according to national law, copy of the opinion or opinions of the ethics committee or committees concerned. Where according to national law the opinion or opinions of the ethics committee or committees is not required at the time of the submission of the application, a copy of the opinion or opinions shall be submitted as soon as available. 4.3. Proof of insurance cover or indemnification of subjects in case of injury, pursuant to Article 69 and the corresponding national law. 4.4. Documents to be used to obtain informed consent, including the patient information sheet and the informed consent document. 4.5. Description of the arrangements to comply with the applicable rules on the protection and confidentiality of personal data, in particular: — organisational and technical arrangements that will be implemented to avoid unauthorised access, disclosure, dissemination, alteration or loss of information and personal data processed; — a description of measures that will be implemented to ensure confidentiality of records and personal data of subjects; and — a description of measures that will be implemented in case of a data security breach in order to mitigate the possible adverse effects. 4.6.Full details of the available technical documentation, for example detailed risk analysis/management documentation or specific test reports, shall, upon request, be submitted to the competent authority reviewing an application.
CHAPTER III OTHER OBLIGATIONS OF THE SPONSOR
1. The sponsor shall undertake to keep available for the competent national authorities any documentation necessary to provide evidence for the documentation referred to in Chapter II of this Annex. If the sponsor is not the natural or legal person responsible for the manufacture of the investigational device, that obligation may be fulfilled by that person on behalf of the sponsor. 2. The Sponsor shall have an agreement in place to ensure that any serious adverse events or any other event as referred to in Article 80(2) are reported by the investigator or investigators to the sponsor in a timely manner. 3. The documentation mentioned in this Annex shall be kept for a period of at least 10 years after the clinical investigation with the device in question has ended, or, in the event that the device is subsequently placed on the market, at least 10 years after the last device has been placed on the market. In the case of implantable devices, the period shall be at least 15 years. Each Member State shall require that this documentation is kept at the disposal of the competent authorities for the period referred to in the first subparagraph in case the sponsor, or its contact person or legal representative as referred to in Article 62(2) established within its territory, goes bankrupt or ceases its activity prior to the end of this period. 4.The Sponsor shall appoint a monitor that is independent from the investigational site to ensure that the investigation is conducted in accordance with the CIP, the principles of good clinical practice and this Regulation. 5. The Sponsor shall complete the follow-up of investigation subjects. 6. The Sponsor shall provide evidence that the investigation is being conducted in line with good clinical practice, for instance through internal or external inspection. 7. The Sponsor shall prepare a clinical investigation report which includes at least the following: — Cover/introductory page or pages indicating the title of the investigation, the investigational device, the single identification number, the CIP number and the details with signatures of the coordinating investigators and the principal investigators from each investigational site. — Details of the author and date of the report. —A summary of the investigation covering the title, purpose of the investigation, description of the investigation, investigational design and methods used, the results of the investigation and conclusion of the investigation. The completion date of the investigation, and in particular details of early termination, temporary halts or suspensions of investigations. — Investigational device description, in particular clearly defined intended purpose. — A summary of the clinical investigation plan covering objectives, design, ethical aspects, monitoring and quality measures, selection criteria, target patient populations, sample size, treatment schedules, follow-up duration, concomitant treatments, statistical plan, including hypothesis, sample size calculation and analysis methods, as well as a justification. — Results of the clinical investigation covering, with rationale and justification, subject demographics, analysis of results related to chosen endpoints, details of subgroup analysis, as well as compliance with the CIP, and covering follow-up of missing data and of patients withdrawing from the clinical investigation, or lost to follow-up. — Summary of serious adverse events, adverse device effects, device deficiencies and any relevant corrective actions. — Discussion and overall conclusions covering safety and performance results, assessment of risks and clinical benefits, discussion of clinical relevance in accordance with clinical state of the art, any specific precautions for specific patient populations, implications for the investigational device, limitations of the investigation.
OTHER LEGAL ISSUES FOR CLINICAL EVALUATION IN MDR
Out of Annex II of MDR
1. DEVICE DESCRIPTION AND SPECIFICATION, INCLUDING VARIANTS AND ACCESSORIES 1.1. Device description and specification (a) product or trade name and a general description of the device including its intended purpose and intended users; (b) the Basic UDI-DI as referred to in Part C of Annex VI assigned by the manufacturer to the device in question, as soon as identification of this device becomes based on a UDI system, or otherwise a clear identification by means of product code, catalogue number or other unambiguous reference allowing traceability; (c) the intended patient population and medical conditions to be diagnosed, treated and/or monitored and other considerations such as patient selection criteria, indications, contra-indications, warnings; (d) principles of operation of the device and its mode of action, scientifically demonstrated if necessary; (e) the rationale for the qualification of the product as a device; (f) the risk class of the device and the justification for the classification rule(s) applied in accordance with Annex VIII; (g) an explanation of any novel features; (h) a description of the accessories for a device, other devices and other products that are not devices, which are intended to be used in combination with it; (i) a description or complete list of the various configurations/variants of the device that are intended to be made available on the market; (j) a general description of the key functional elements, e.g. its parts/components (including software if appropriate), its formulation, its composition, its functionality and, where relevant, its qualitative and quantitative composition. Where appropriate, this shall include labelled pictorial representations (e.g. diagrams, photographs, and drawings), clearly indicating key parts/components, including sufficient explanation to understand the drawings and diagrams; (k) a description of the raw materials incorporated into key functional elements and those making either direct contact with the human body or indirect contact with the body, e.g., during extracorporeal circulation of body fluids; (l) technical specifications, such as features, dimensions and performance attributes, of the device and any variants/configurations and accessories that would typically appear in the product specification made available to the user, for example in brochures, catalogues and similar publications.
1.2. Reference to previous and similar generations of the device (a) an overview of the previous generation or generations of the device produced by the manufacturer, where such devices exist; (b) an overview of identified similar devices available on the Union or international markets, where such devices exist.
Out of MEDDEV 2.7.1 rev. 4
Appendix A3. Device description - typical contents
The description should be detailed enough to allow for a valid evaluation of the state of compliance
with Essential Requirements, the retrieval of meaningful literature data and, if applicable, the
assessment of equivalence to other devices described in the scientific literature:
* name, models, sizes, components of the device, including software and accessories
* device group to which the device belongs (e.g. biological artificial aortic valve)
* whether the device is being developed/ undergoing initial CE-marking/ is CE-marked
* whether the device is currently on the market in Europe or in other countries, since when, number of devices placed on the market
* intended purpose of the device
* exact medical indications (if applicable)
* name of disease or condition/ clinical form, stage, severity/ symptoms or aspects to be treated, managed or diagnosed
* patient populations (adults / children / infants, other aspects)
* intended user (use by health care professional / lay person)
* organs / parts of the body / tissues or body fluids contacted by the device
* duration of use or contact with the body
* repeat applications, including any restrictions as to the number or duration of reapplications
* contact with mucosal membranes/ invasiveness/ implantation
* contraindications
* precautions required by the manufacturer
* single use / reusable
* other aspects
* general description of the medical device including
* a concise physical and chemical description
* the technical specifications, mechanical characteristics
* sterility
* radioactivity
* how the device achieves its intended purpose
* principles of operation
* materials used in the device with focus on materials coming in contact (directly or indirectly) with the patient/ user, description of body parts concerned
* whether it incorporates a medicinal substance (already on the market or new), animal tissues, or blood components, the purpose of the component
* other aspects
* whether the device is intended to cover medical needs that are otherwise unmet/ if there are medical alternatives to the device / if the device is equivalent to an existing device, with a description of the situation and any new features
* if the device is intended to enter the market based on equivalence:
* name, models, sizes, settings components of the device presumed to be equivalent, including software and accessories
* whether equivalence has already been demonstrated
* Intended performance, including the technical performance of the device intended by the manufacturer, the intended clinical benefits, claims regarding clinical performance and clinical safety that the manufacturer intends to use
* For devices based on predecessor devices: Name, models, sizes of the predecessor device, whether the predecessor device is still on the market, description of the modifications, date of the modifications.
* The current version number or date of the information materials supplied by the vmanufacturer (label, IFU, available promotional materials and accompanying documents possibly foreseen by the manufacturer).
Notified Bodies and of Conformity Assessment: Clinical Aspects
Requirements for NBs in connection with clinical evaluation:
Article 36 Requirements relating to notified bodies
1.Notified bodies shall fulfil the tasks for which they are designated in accordance with this Regulation. They shall satisfy the organisational and general requirements and the quality management, resource and process requirements that are necessary to fulfil those tasks. In particular, notified bodies shall comply with Annex VII. In order to meet the requirements referred to in the first subparagraph, notified bodies shall have permanent availability of sufficient administrative, technical and scientific personnel in accordance with Section 3.1.1 of Annex VII and personnel with relevant clinical expertise in accordance with Section 3.2.4 of Annex VII, where possible employed by the notified body itself.
Article 45 Review of notified body assessment of technical documentation and clinical evaluation documentation
1.The authority responsible for notified bodies, as part of its ongoing monitoring of notified bodies, shall review an appropriate number of notified body assessments of manufacturers' technical documentation, in particular the clinical evaluation documentation as referred to in points (c) and (d) of Section 6.1 of Annex II to verify the conclusions drawn by the notified body based on the information presented by the manufacturer. The reviews by the authority responsible for notified bodies shall be conducted both off-site and on-site. 2.The sampling of files to be reviewed in accordance with paragraph 1 shall be planned and representative of the types and risk of devices certified by the notified body, in particular high-risk devices, and be appropriately justified and documented in a sampling plan, which shall be made available by the authority responsible for notified bodies to the MDCG upon request. 3.The authority responsible for notified bodies shall review whether the assessment by the notified body was conducted appropriately and shall check the procedures used, associated documentation and the conclusions drawn by the notified body. Such checking shall include the technical documentation and clinical evaluation documentation of the manufacturer upon which the notified body has based its assessment. Such reviews shall be conducted utilising CS. 4.Those reviews shall also form part of the re-assessment of notified bodies in accordance with Article 44(10) and the joint assessment activities referred to in Article 47(3). The reviews shall be conducted utilising appropriate expertise. 5.Based on the reports of the reviews and assessments by the authority responsible for notified bodies or joint assessment teams, on input from the market surveillance, vigilance and post-market surveillance activities described in Chapter VII, on the continuous monitoring of technical progress, or on the identification of concerns and emerging issues concerning the safety and performance of devices, the MDCG may recommend that the sampling, carried out under this Article, cover a greater or lesser proportion of the technical documentation and clinical evaluation documentation assessed by a notified body. 6.The Commission may, by means of implementing acts, adopt measures setting out the detailed arrangements, associated documents for, and coordination of, the review of assessments of technical documentation and clinical evaluation documentation, as referred to in this Article. Those implementing acts shall be adopted in accordance with the examination procedure referred to in Article 114(3).
ANNEX VII REQUIREMENTS TO BE MET BY NOTIFIED BODIES
3.1.1.: Notified bodies shall be capable of carrying out all the tasks falling to them under this Regulation with the highest degree of professional integrity and the requisite competence in the specific field, whether those tasks are carried out by notified bodies themselves or on their behalf and under their responsibility. In particular, notified bodies shall have the necessary personnel and possess or have access to all equipment, facilities and competence needed to perform properly the technical, scientific and administrative tasks entailed in the conformity assessment activities in relation to which they have been designated. Such requirement presupposes at all times and for each conformity assessment procedure and each type of devices in relation to which they have been designated, that the notified body has permanent availability of sufficient administrative, technical and scientific personnel who possess experience and knowledge relating to the relevant devices and the corresponding technologies. Such personnel shall be in sufficient numbers to ensure that the notified body in question can perform the conformity assessment tasks, including the assessment of the medical functionality, clinical evaluations and the performance and safety of devices, for which it has been designated, having regard to the requirements of this Regulation, in particular, those set out in Annex I. A notified body's cumulative competences shall be such as to enable it to assess the types of devices for which it is designated. The notified body shall have sufficient internal competence to critically evaluate assessments conducted by external expertise. Tasks which a notified body is precluded from subcontracting are set out in Section 4.1.
3.2. Qualification criteria in relation to personnel 3.2.1. The Notified Body shall establish and document qualification criteria and procedures for selection and authorisation of persons involved in conformity assessment activities, including as regards knowledge, experience and other competence required, and the required initial and ongoing training. The qualification criteria shall address the various functions within the conformity assessment process, such as auditing, product evaluation or testing, technical documentation review and decision-making, as well as the devices, technologies and areas, such as biocompatibility, sterilisation, tissues and cells of human and animal origin and clinical evaluation, covered by the scope of designation. 3.2.2. The qualification criteria referred to in Section 3.2.1 shall refer to the scope of a notified body's designation in accordance with the scope description used by the Member State for the notification referred to in Article 42(3), providing a sufficient level of detail for the required qualification within the subdivisions of the scope description. Specific qualification criteria shall be defined at least for the assessment of: — the pre-clinical evaluation, — clinical evaluation, — tissues and cells of human and animal origin, — functional safety, — software, — packaging, — devices that incorporate as an integral part a medicinal product, — devices that are composed of substances or of combinations of substances that are absorbed by or locally dispersed in the human body and — the different types of sterilisation processes. 3.2.4. The notified body shall have permanent availability of personnel with relevant clinical expertise and where possible such personnel shall be employed by the notified body itself. Such personnel shall be integrated throughout the notified body's assessment and decision making process in order to: — identify when specialist input is required for the assessment of the clinical evaluation conducted by the manufacturer and identify appropriately qualified experts; — appropriately train external clinical experts in the relevant requirements of this Regulation, CS, guidance and harmonised standards and ensure that the external clinical experts are fully aware of the context and implications of their assessment and the advice they provide; — be able to review and scientifically challenge the clinical data contained within the clinical evaluation, and any associated clinical investigations, and appropriately guide external clinical experts in the assessment of the clinical evaluation presented by the manufacturer; — be able to scientifically evaluate and, if necessary, challenge the clinical evaluation presented, and the results of the external clinical experts' assessment of the manufacturer's clinical evaluation; — be able to ascertain the comparability and consistency of the assessments of clinical evaluations conducted by clinical experts; — be able to make an assessment of the manufacturer's clinical evaluation and a clinical judgement of the opinion provided by any external expert and make a recommendation to the notified body's decision maker; and — be able to draw up records and reports demonstrating that the relevant conformity assessment activities have been appropriately carried out.
3.2.5. The personnel responsible for carrying out product-related reviews (product reviewers), such as technical documentation reviews or
type examination, including aspects such as clinical evaluation, biological safety, sterilisation and software validation, shall have all of
the following proven qualifications:
— successful completion of a university or a technical college degree or equivalent qualification in relevant studies, e.g. medicine,
pharmacy, engineering or other relevant sciences;
— four years' professional experience in the field of healthcare products or related activities, such as in manufacturing, auditing or
research, of which two years shall be in the design, manufacture, testing or use of the device or technology to be assessed or related to the
scientific aspects to be assessed;
— knowledge of device legislation, including the general safety and performance requirements set out in Annex I;
— appropriate knowledge and experience of relevant harmonised standards, CS and guidance documents;
— appropriate knowledge and experience of risk management and related device standards and guidance documents;
— appropriate knowledge and experience of clinical evaluation;
— appropriate knowledge of the devices which they are assessing;
— appropriate knowledge and experience of the conformity assessment procedures laid down in Annexes IX to XI, in particular of the
aspects of those procedures for which they are responsible, and adequate authorisation for carrying out those assessments;
— the ability to draw up records and reports demonstrating that the relevant conformity assessment activities have been appropriately
carried out.
4.5. Conformity assessment activities 4.5.1. General The notified body and its personnel shall carry out the conformity assessment activities with the highest degree of professional integrity and the requisite technical and scientific competence in the specific fields. The notified body shall have expertise, facilities and documented procedures that are sufficient to effectively conduct the conformity assessment activities for which the notified body in question is designated, taking account of the relevant requirements set out in Annexes IX to XI, and in particular all of the following requirements: … — review the manufacturer's procedures and documentation relating to clinical evaluation, — address the interface between the manufacturer's risk management process and its appraisal and analysis of the pre-clinical and clinical evaluation and to evaluate their relevance for the demonstration of conformity with the relevant requirements in Annex I, — carry out the specific procedures referred to in Sections 5.2 to 5.4 of Annex IX, —in the case of class IIa or class IIb devices, assess the technical documentation of devices selected on a representative basis, … — evaluate and verify a manufacturer's compliance with relevant Annexes. The notified body shall, where relevant, take into consideration available CS, guidance and best practice documents and harmonised standards, even if the manufacturer does not claim to be in compliance.
4.5.2. Quality management system auditing … (b) Based on the audit programme it has drawn up, the notified body shall, in accordance with its documented procedures: — audit the manufacturer's quality management system, in order to verify that the quality management system ensures that the devices covered conform to the relevant provisions of this Regulation which apply to devices at every stage, from design through final quality control to ongoing surveillance, and shall determine whether the requirements of this Regulation are met, — based on relevant technical documentation and in order to determine whether the manufacturer meets the requirements referred to in the relevant conformity assessment Annex, review and audit the manufacturer's processes and subsystems, in particular for: — design and development, — production and process controls, — product documentation, — purchasing controls including verification of purchased devices, — corrective and preventive actions, including for post-market surveillance, and — PMCF, and review and audit requirements and provisions adopted by the manufacturer, including those in relation to fulfilling the general safety and performance requirements set out in Annex I. The documentation shall be sampled in such a manner as to reflect the risks associated with the intended use of the device, the complexity of the manufacturing technologies, the range and classes of devices produced and any available post-market surveillance information, — if not already covered by the audit programme, audit the control of processes on the premises of the manufacturer's suppliers, when the conformity of finished devices is significantly influenced by the activity of suppliers and, in particular when the manufacturer cannot demonstrate sufficient control over its suppliers, — conduct assessments of the technical documentation based on its sampling plan and taking account of Sections 4.5.4. and 4.5.5. for pre clinical and clinical evaluations, and — the notified body shall ensure that audit findings are appropriately and consistently classified in accordance with the requirements of this Regulation and with relevant standards, or with best practice documents developed or adopted by the MDCG.
4.5.3. Product verification Assessment of the technical documentation For assessment of the technical documentation conducted in accordance with Chapter II of Annex IX, notified bodies shall have sufficient expertise, facilities and documented procedures for: — the allocation of appropriately qualified and authorised personnel for the examination of individual aspects such as use of the device, biocompatibility, clinical evaluation, risk management, and sterilisation, and — the assessment of conformity of the design with this Regulation, and for taking account of Sections 4.5.4. to 4.5.6. That assessment shall include examination of the implementation by manufacturers of incoming, in- process and final checks and the results thereof. If further tests or other evidence is required for the assessment of conformity with the requirements of this Regulation, the notified body in question shall carry out adequate physical or laboratory tests in relation to the device or request the manufacturer to carry out such tests.
4.5.5. Clinical evaluation assessment The notified body shall have documented procedures in place relating to the assessment of a manufacturer's procedures and documentation relating to clinical evaluation both for initial conformity assessment and on an ongoing basis. The notified body shall examine, validate and verify that manufacturers' procedures and documentation adequately address: — the planning, conduct, assessment, reporting and updating of the clinical evaluation as referred to in Annex XIV, — post-market surveillance and PMCF, — the interface with the risk management process, — the appraisal and analysis of the available data and its relevance with regard to demonstrating conformity with the relevant requirements in Annex I, and — the conclusions drawn with regard to the clinical evidence and drawing up of the clinical evaluation report. These procedures referred to in the first paragraph shall take into consideration available CS, guidance and best practice documents. The notified body's assessment of clinical evaluations as referred to in Annex XIV shall cover: — the intended use specified by the manufacturer and claims for the device defined by it, — the planning of the clinical evaluation, — the methodology for the literature search, — relevant documentation from the literature search, — the clinical investigation, — validity of equivalence claimed in relation to other devices, the demonstration of equivalence, the suitability and conclusions data from equivalent and similar devices, — post-market surveillance and PMCF, — the clinical evaluation report, and — justifications in relation to non-performance of clinical investigations or PMCF. In relation to clinical data from clinical investigations included within the clinical evaluation, the notified body in question shall ensure that the conclusions drawn by the manufacturer are valid in the light of the approved clinical investigation plan. The notified body shall ensure * that the clinical evaluation adequately addresses the relevant safety and performance requirements provided for in Annex I, * that it is appropriately aligned with the risk management requirements, * that it is conducted in accordance with Annex XIV and * that it is appropriately reflected in the information provided relating to the device.
4.6. Reporting The notified body shall: — … — clearly document the conclusions of its assessment of clinical evaluation in a clinical evaluation assessment report, and
4.8. Decisions and Certifications The notified body shall have documented procedures for decision-making including as regards the allocation of responsibilities for the issuance, suspension, restriction and withdrawal of certificates. Those procedures shall include the notification requirements laid down in Chapter V of this Regulation. The procedures shall allow the notified body in question to: — decide, based on the assessment documentation and additional information available, whether the requirements of this Regulation are fulfilled, — decide, based on the results of its assessment of the clinical evaluation and risk management, whether the post-market surveillance plan, including the PMCF plan, is adequate, — decide on specific milestones for further review by the notified body of the up to date clinical evaluation, — decide whether specific conditions or provisions need to be defined for the certification, — decide, based on the novelty, risk classification, clinical evaluation and conclusions from the risk analysis of the device, on a period of certification not exceeding five years,
4.10. Surveillance activities and post-certification monitoring The notified body shall have documented procedures: — defining how and when surveillance activities of manufacturers are to be conducted. Those procedures shall include arrangements for unannounced on-site audits of manufacturers and, where applicable, subcontractors and suppliers carrying out product tests and the monitoring of compliance with any conditions binding manufacturers and associated with certification decisions, such as updates to clinical data at defined intervals, — for screening relevant sources of scientific and clinical data and post-market information relating to the scope of their designation. Such information shall be taken into account in the planning and conduct of surveillance activities, and
The notified body shall, if listed as part of the conditions for certification: — conduct an in-depth review of the clinical evaluation as most recently updated by the manufacturer based on the manufacturer's post-market surveillance, on its PMCF and on clinical literature relevant to the condition being treated with the device or on clinical literature relevant to similar devices, — clearly document the outcome of the in-depth review and address any specific concerns to the manufacturer or impose any specific conditions on it, and — ensure that the clinical evaluation as most recently updated, is appropriately reflected in the instructions for use and, where applicable, the summary of safety and performance.
4.11. Re-certification The notified body shall have documented procedures in place relating to the re-certification reviews and the renewal of certificates. Re certification of approved quality management systems or EU technical documentation assessment certificates or EU type-examination certificates shall occur at least every five years. The notified body shall have documented procedures relating to renewals of EU technical documentation assessment certificates and EU type-examination certificates and those procedures shall require the manufacturer in question to submit a summary of changes and scientific findings for the device, including: (a) all changes to the originally approved device, including changes not yet notified, (b) experience gained from post-market surveillance, (c) experience from risk management, (d) experience from updating the proof of compliance with the general safety and performance requirements set out in Annex I, (e) experience from reviews of the clinical evaluation, including the results of any clinical investigations and PMCF, (f) changes to the requirements, to components of the device or to the scientific or regulatory environment, (g) changes to applied or new harmonised standards, CS or equivalent documents, and (h) changes in medical, scientific and technical knowledge, such as: — new treatments, — changes in test methods, — new scientific findings on materials and components, including findings on their biocompatibility, — experience from studies on comparable devices, — data from registers and registries, — experience from clinical investigations with comparable devices. The notified body shall have documented procedures to assess the information referred to in the second paragraph and shall pay particular attention to clinical data from post-market surveillance and PMCF activities undertaken since the previous certification or re-certification, including appropriate updates to manufacturers' clinical evaluation reports. For the decision on re-certification, the notified body in question shall use the same methods and principles as for the initial certification decision. If necessary, separate forms shall be established for re-certification taking into account the steps taken for certification such as application and application review.
Conformity assessment: Please note that for certain high-risk MDs there is in Art. 54 an additional consultation procedure concerning clinical evaluation and its assessment by the NB, where an expert panel is asked for a scientific opinion during conformity assessment. Art. 54 has to be read in conjunction with Annex IX.II.5.1., which is also referenced in Annex X.6. of MDR: “Art 54 Clinical evaluation consultation procedure for certain class III and class IIb devices 1. In addition to the procedures applicable pursuant to Article 52 , a notified body shall also follow the procedure regarding clinical evaluation consultation as specified in Section 5.1 of Annex IX or as referred to in Section 6 of Annex X , as applicable, when performing a conformity assessment of the following devices: (a) class III implantable devices, and (b) class IIb active devices intended to administer and/or remove a medicinal product, as referred to in Section 6.4 of Annex VIII (Rule 12). 2. The procedure referred to in paragraph 1 shall not be required for the devices referred to therein: (a) in the case of renewal of a certificate issued under this Regulation; (b) where the device has been designed by modifying a device already marketed by the same manufacturer for the same intended purpose, provided that the manufacturer has demonstrated to the satisfaction of the notified body that the modifications do not adversely affect the benefit-risk ratio of the device; or (c) where the principles of the clinical evaluation of the device type or category have been addressed in a CS referred to in Article 9 and the notified body confirms that the clinical evaluation of the manufacturer for this device is in compliance with the relevant CS for clinical evaluation of that kind of device. 3. The notified body shall notify the competent authorities, the authority responsible for notified bodies and the Commission through the electronic system referred to in Article 57 of whether or not the procedure referred to in paragraph 1 of this Article is to be applied. That notification shall be accompanied by the clinical evaluation assessment report. 4. The Commission shall draw up an annual overview of devices which have been subject to the procedure specified in Section 5.1 of Annex IX and referred to in Section 6 of Annex X. The annual overview shall include the notifications in accordance with paragraph 3 of this Article and point (e) of Section 5.1 of Annex IX and a listing of the cases where the notified body did not follow the advice from the expert panel. The Commission shall submit this overview to the European Parliament, to the Council and to the MDCG. 5. The Commission shall by 27 May 2025 draw up a report on the operation of this Article and submit it to the European Parliament and to the Council. The report shall take into account the annual overviews and any available relevant recommendations from the MDCG. On the basis of that report the Commission shall, if appropriate, make proposals for amendments to this Regulation.
ANNEX IX.II.5.1. Assessment procedure for certain class III and class IIb devices
(a) For class III implantable devices, and for class IIb active devices intended to administer and/or remove a medicinal product as referred to in Section 6.4. of Annex VIII (Rule 12), the notified body shall, having verified the quality of clinical data supporting the clinical evaluation report of the manufacturer referred to in Article 61(12), prepare a clinical evaluation assessment report which sets out its conclusions concerning the clinical evidence provided by the manufacturer, in particular concerning the benefit-risk determination, the consistency of that evidence with the intended purpose, including the medical indication or indications and the PMCF plan referred to in Article 10(3) and Part B of Annex XIV. The notified body shall transmit its clinical evaluation assessment report, along with the manufacturer's clinical evaluation documentation, referred to in points (c) and (d) of Section 6.1 of Annex II, to the Commission. The Commission shall immediately transmit those documents to the relevant expert panel referred to in Article 106. (b) The notified body may be requested to present its conclusions as referred to in point (a) to the expert panel concerned. (c) The expert panel shall decide, under the supervision of the Commission, on the basis of all of the following criteria: i. the novelty of the device or of the related clinical procedure involved, and the possible major clinical or health impact thereof; ii. a significantly adverse change in the benefit-risk profile of a specific category or group of devices due to scientifically valid health concerns in respect of components or source material or in respect of the impact on health in the case of failure of the device; iii. a significantly increased rate of serious incidents reported in accordance with Article 87 in respect of a specific category or group of devices, whether to provide a scientific opinion on the clinical evaluation assessment report of the notified body based on the clinical evidence provided by the manufacturer, in particular concerning the benefit-risk determination, the consistency of that evidence with the medical indication or indications and the PMCF plan. That scientific opinion shall be provided within a period of 60 days, starting on the day of receipt of the documents from the Commission as referred to in point (a). The reasons for the decision to provide a scientific opinion on the basis of the criteria in points (i), (ii) and (iii) shall be included in the scientific opinion. Where the information submitted is not sufficient for the expert panel to reach a conclusion, this shall be stated in the scientific opinion. (d) The expert panel may decide, under the supervision of the Commission, on the basis of the criteria laid down in point (c) not to provide a scientific opinion, in which case it shall inform the notified body as soon as possible and in any event within 21 days of receipt of the documents as referred to in point (a) from the Commission. The expert panel shall within that time limit provide the notified body and the Commission with the reasons for its decision, whereupon the notified body may proceed with the certification procedure of that device. (e) The expert panel shall within 21 days of receipt of the documents from the Commission notify the Commission, through Eudamed whether it intends to provide a scientific opinion, pursuant to point (c), or whether it intends not to provide a scientific opinion, pursuant to point (d). (f) Where no opinion has been delivered within a period of 60 days, the notified body may proceed with the certification procedure of the device in question. (g) The notified body shall give due consideration to the views expressed in the scientific opinion of the expert panel. Where the expert panel finds that the level of clinical evidence is not sufficient or otherwise gives rise to serious concerns about the benefit-risk determination, the consistency of that evidence with the intended purpose, including the medical indication(s), and with the PMCF plan, the notified body shall, if necessary, advise the manufacturer to restrict the intended purpose of the device to certain groups of patients or certain medical indications and/or to impose a limit on the duration of validity of the certificate, to undertake specific PMCF studies, to adapt the instructions for use or the summary of safety and performance, or to impose other restrictions in its conformity assessment report, as appropriate. The notified body shall provide a full justification where it has not followed the advice of the expert panel in its conformity assessment report and the Commission shall without prejudice to Article 109 make both the scientific opinion of the expert panel and the written justification provided by the notified body publicly available via Eudamed. (h) The Commission, after consultation with the Member States and relevant scientific experts shall provide guidance for expert panels for consistent interpretation of the criteria in point (c) before 26 May 2020.
ANNEX IX CONFORMITY ASSESSMENT BASED ON A QUALITY MANAGEMENT SYSTEM AND ON ASSESSMENT OF TECHNICAL DOCUMENTATION
CHAPTER I QUALITY MANAGEMENT SYSTEM
Please remember that the clinical evaluation, incl. PMCF, as given in Art. 61 and Annex XIV, is an obligatory life-cycle process any manufacturer has to perform under its obligatory QMS! (see Art. 10 of MDR) Application for QMS Assessment:
2.1. … “— the documentation on the manufacturer's post-market surveillance system and, where applicable, on the PMCF plan, and the procedures put in place to ensure compliance with the obligations resulting from the provisions on vigilance set out in Articles 87 to 92, — a description of the procedures in place to keep up to date the post-market surveillance system, and, where applicable, the PMCF plan, and the procedures ensuring compliance with the obligations resulting from the provisions on vigilance set out in Articles 87 to 92, as well as the undertaking by the manufacturer to apply those procedures, — documentation on the clinical evaluation plan, and — a description of the procedures in place to keep up to date the clinical evaluation plan, taking into account the state of the art.” …
2.2. Implementation of the QMS: … “(c) the procedures and techniques for monitoring, verifying, validating and controlling the design of the devices and the corresponding documentation as well as the data and records arising from those procedures and techniques. Those procedures and techniques shall specifically cover: … — the clinical evaluation, pursuant to Article 61 and Annex XIV, including post-market clinical follow-up,” “In addition, the manufacturer shall grant the notified body access to the technical documentation referred to in Annexes II and III.”
2.3. Audits: … “Moreover, in the case of class IIa and class IIb devices, the quality management system assessment shall be accompanied by the assessment of technical documentation for devices selected on a representative basis in accordance with Sections 4.4 to 4.8.” These sections are closely related to the NBs assessment of clinical evaluation!
3. Surveillance assessment applicable to class IIa, class IIb and class III devices … 3.2. … “— documentation on any findings and conclusions resulting from the application of the post-market surveillance plan, including the PMCF plan, for a representative sample of devices, and of the provisions on vigilance set out in Articles 87 to 92,” … “3.5. In the case of class IIa and class IIb devices, the surveillance assessment shall also include an assessment of the technical documentation as referred to in Sections 4.4 to 4.8 for the device or devices concerned on the basis of further representative samples chosen in accordance with the rationale documented by the notified body in accordance with the second paragraph of Section 2.3.”
“Chapter II: ASSESSMENT OF THE TECHNICAL DOCUMENTATION 4. Assessment of the technical documentation applicable to class III devices and to the class IIb devices referred to in the second subparagraph of Article 52(4) 4.1. In addition to the obligations laid down in Section 2, the manufacturer shall lodge with the notified body an application for assessment of the technical documentation relating to the device which it plans to place on the market or put into service and which is covered by the quality management system referred to in Section 2. 4.2. The application shall describe the design, manufacture and performance of the device in question. It shall include the technical documentation as referred to in Annexes II and III. 4.3. The notified body shall examine the application by using staff, employed by it, with proven knowledge and experience regarding the technology concerned and its clinical application. The notified body may require the application to be completed by having further tests carried out or requesting further evidence to be provided to allow assessment of conformity with the relevant requirements of the Regulation. The notified body shall carry out adequate physical or laboratory tests in relation to the device or request the manufacturer to carry out such tests. 4.4. The notified body shall review the clinical evidence presented by the manufacturer in the clinical evaluation report and the related clinical evaluation that was conducted. The notified body shall employ device reviewers with sufficient clinical expertise and, if necessary, use external clinical experts with direct and current experience relating to the device in question or the clinical condition in which it is utilised, for the purposes of that review. 4.5. The notified body shall, in circumstances in which the clinical evidence is based partly or totally on data from devices which are claimed to be equivalent to the device under assessment, assess the suitability of using such data, taking into account factors such as new indications and innovation. The notified body shall clearly document its conclusions on the claimed equivalence, and on the relevance and adequacy of the data for demonstrating conformity. For any characteristic of the device claimed as innovative by the manufacturer or for new indications, the notified body shall assess to what extent specific claims are supported by specific pre-clinical and clinical data and risk analysis. 4.6. The notified body shall verify that the clinical evidence and the clinical evaluation are adequate and shall verify the conclusions drawn by the manufacturer on the conformity with the relevant general safety and performance requirements. That verification shall include consideration of the adequacy of the benefit-risk determination, the risk management, the instructions for use, the user training and the manufacturer's post-market surveillance plan, and include a review of the need for, and the adequacy of, the PMCF plan proposed, where applicable. 4.7. Based on its assessment of the clinical evidence, the notified body shall consider the clinical evaluation and the benefit-risk determination, and whether specific milestones need to be defined to allow the notified body to review updates to the clinical evidence that result from post-market surveillance and PMCF data. 4.8. The notified body shall clearly document the outcome of its assessment in the clinical evaluation assessment report. 4.9. The notified body shall provide the manufacturer with a report on the technical documentation assessment, including a clinical evaluation assessment report. If the device conforms to the relevant provisions of this Regulation, the notified body shall issue an EU technical documentation assessment certificate. The certificate shall contain the conclusions of the technical documentation assessment, the conditions of the certificate's validity, the data needed for identification of the approved design, and, where appropriate, a description of the intended purpose of the device.”
5.1. Assessment procedure for certain class III and class IIb devices
(a) For class III implantable devices, and for class IIb active devices intended to administer and/or remove a medicinal product as referred
to in Section 6.4. of Annex VIII (Rule 12), the notified body shall, having verified the quality of clinical data supporting the clinical
evaluation report of the manufacturer referred to in Article 61(12), prepare a clinical evaluation assessment report which sets out its
conclusions concerning the clinical evidence provided by the manufacturer, in particular concerning the benefit-risk determination, the
consistency of that evidence with the intended purpose, including the medical indication or indications and the PMCF plan referred to in
Article 10(3) and Part B of Annex XIV. The notified body shall transmit its clinical evaluation assessment report, along with the
manufacturer's clinical evaluation documentation, referred to in points (c) and (d) of Section 6.1 of Annex II, to the Commission. The
Commission shall immediately transmit those documents to the relevant expert panel referred to in Article 106.
(b) The notified body may be requested to present its conclusions as referred to in point (a) to the expert panel concerned.
(c) The expert panel shall decide, under the supervision of the Commission, on the basis of all of the following criteria:
(i) the novelty of the device or of the related clinical procedure involved, and the possible major clinical or health impact thereof;
(ii) a significantly adverse change in the benefit-risk profile of a specific category or group of devices due to scientifically valid
health concerns in respect of components or source material or in respect of the impact on health in the case of failure of the device;
(iii) a significantly increased rate of serious incidents reported in accordance with Article 87 in respect of a specific category or
group of devices,
whether to provide a scientific opinion on the clinical evaluation assessment report of the notified body based on the clinical evidence
provided by the manufacturer, in particular concerning the benefit-risk determination, the consistency of that evidence with the medical
indication or indications and the PMCF plan. That scientific opinion shall be provided within a period of 60 days, starting on the day of
receipt of the documents from the Commission as referred to in point (a). The reasons for the decision to provide a scientific opinion on
the basis of the criteria in points (i), (ii) and (iii) shall be included in the scientific opinion. Where the information submitted is
not sufficient for the expert panel to reach a conclusion, this shall be stated in the scientific opinion.
(d) The expert panel may decide, under the supervision of the Commission, on the basis of the criteria laid down in point (c) not to provide
a scientific opinion, in which case it shall inform the notified body as soon as possible and in any event within 21 days of receipt of the
documents as referred to in point (a) from the Commission. The expert panel shall within that time limit provide the notified body and the
Commission with the reasons for its decision, whereupon the notified body may proceed with the certification procedure of that device.
(e) The expert panel shall within 21 days of receipt of the documents from the Commission notify the Commission, through Eudamed whether it
intends to provide a scientific opinion, pursuant to point (c), or whether it intends not to provide a scientific opinion, pursuant to point
(d).
(f) Where no opinion has been delivered within a period of 60 days, the notified body may proceed with the certification procedure of the
device in question.
(g) The notified body shall give due consideration to the views expressed in the scientific opinion of the expert panel. Where the expert
panel finds that the level of clinical evidence is not sufficient or otherwise gives rise to serious concerns about the benefit-risk
determination, the consistency of that evidence with the intended purpose, including the medical indication(s), and with the PMCF plan, the
notified body shall, if necessary, advise the manufacturer to restrict the intended purpose of the device to certain groups of patients or
certain medical indications and/or to impose a limit on the duration of validity of the certificate, to undertake specific PMCF studies, to
adapt the instructions for use or the summary of safety and performance, or to impose other restrictions in its conformity assessment report,
as appropriate. The notified body shall provide a full justification where it has not followed the advice of the expert panel in its
conformity assessment report and the Commission shall without prejudice to Article 109 make both the scientific opinion of the expert panel
and the written justification provided by the notified body publicly available via Eudamed.
(h) The Commission, after consultation with the Member States and relevant scientific experts shall provide guidance for expert panels for
consistent interpretation of the criteria in point (c) before 26 May 2020.
CHAPTER II ASSESSMENT OF THE TECHNICAL DOCUMENTATION
“4. Assessment of the technical documentation applicable to class III devices and to the class IIb devices referred to in the second subparagraph of Article 52(4). … 4.2. The application shall describe the design, manufacture and performance of the device in question. It shall include the technical documentation as referred to in Annexes II and III. … 4.4. The notified body shall review the clinical evidence presented by the manufacturer in the clinical evaluation report and the related clinical evaluation that was conducted. The notified body shall employ device reviewers with sufficient clinical expertise and, if necessary, use external clinical experts with direct and current experience relating to the device in question or the clinical condition in which it is utilised, for the purposes of that review. 5.5.2017 L 117/149 Official Journal of the European Union EN 4.5. The notified body shall, in circumstances in which the clinical evidence is based partly or totally on data from devices which are claimed to be equivalent to the device under assessment, assess the suitability of using such data, taking into account factors such as new indications and innovation. The notified body shall clearly document its conclusions on the claimed equivalence, and on the relevance and adequacy of the data for demonstrating conformity. For any characteristic of the device claimed as innovative by the manufacturer or for new indications, the notified body shall assess to what extent specific claims are supported by specific pre-clinical and clinical data and risk analysis. 4.6. The notified body shall verify that the clinical evidence and the clinical evaluation are adequate and shall verify the conclusions drawn by the manufacturer on the conformity with the relevant general safety and performance requirements. That verification shall include consideration of the adequacy of the benefit-risk determination, the risk management, the instructions for use, the user training and the manufacturer's post-market surveillance plan, and include a review of the need for, and the adequacy of, the PMCF plan proposed, where applicable. 4.7. Based on its assessment of the clinical evidence, the notified body shall consider the clinical evaluation and the benefit-risk determination, and whether specific milestones need to be defined to allow the notified body to review updates to the clinical evidence that result from post-market surveillance and PMCF data. 4.8. The notified body shall clearly document the outcome of its assessment in the clinical evaluation assessment report. 4.9. The notified body shall provide the manufacturer with a report on the technical documentation assessment, including a clinical evaluation assessment report. If the device conforms to the relevant provisions of this Regulation, the notified body shall issue an EU technical documentation assessment certificate. The certificate shall contain the conclusions of the technical documentation assessment, the conditions of the certificate's validity, the data needed for identification of the approved design, and, where appropriate, a description of the intended purpose of the device.”
5. Specific additional procedures
ANNEX X CONFORMITY ASSESSMENT BASED ON TYPE-EXAMINATION
… 2. Application The application for type-examination contains the technical documentation acc. to Annex II and III and therefore the clinical evaluation report and annexed materials and the PMCF-plan and PMCF evaluation reports
3. Assessment The notified body shall: (a) examine the application by using staff with proven knowledge and experience regarding the technology concerned and its clinical application. … (c) review the clinical evidence presented by the manufacturer in the clinical evaluation report in accordance with Section 4 of Annex XIV. The notified body shall employ device reviewers with sufficient clinical expertise and, if necessary, use external clinical experts with direct and current experience relating to the device in question or to the clinical condition in which it is utilised, for the purposes of that review; (d) in circumstances in which the clinical evidence is based partly or totally on data from devices which are claimed to be similar or equivalent to the device under assessment, assess the suitability of using such data, taking into account factors such as new indications and innovation. The notified body shall clearly document its conclusions on the claimed equivalence, and on the relevance and adequacy of the data for demonstrating conformity; (e) clearly document the outcome of its assessment in a pre-clinical and clinical evaluation assessment report as part of the EU type examination report referred to in point (i);
6. Specific additional procedures “Section 5 of Annex IX shall apply with the proviso that any reference to an EU technical documentation assessment certificate shall be understood as a reference to an EU type-examination certificate.” See consultation procedure under Section 5.1. of Annex IX above.
ANNEX XI, PART A PRODUCTION QUALITY ASSURANCE
7. Surveillance … “— documentation on any findings and conclusions resulting from the application of the post-market surveillance plan, including the PMCF plan, for a representative sample of devices, and of the provisions on vigilance set out in Articles 87 to 92,” (= reference to Annex IX, 3.2. … 10. Application to class IIa devices The technical documentation acc. to Annexes II and III (including the part on clinical evaluation!) of representative samples must be in compliance with the MDR.
ANNEX XI, PART B PRODUCT VERIFICATION
“13. The manufacturer shall undertake to institute and keep up to date a post-market surveillance plan, including a PMCF plan, and the procedures ensuring compliance with the obligations of the manufacturer resulting from the provisions on vigilance and post-market surveillance system set out in Chapter VII.” …
GSPR
Clinical evaluation shall form an obligatory part of the demonstration of compliance with the applicable general requirements for the safety and performance of MPs as set out in Annex I. The manufacturer shall, on the basis of the intended purpose of the medical device as indicated by him, make his claims in order to comply with the essential safety and performance requirements of Annex I. - the safety and efficacy/effectiveness of the medical device and an - acceptable benefit/risk ratio in comparison to the currently recognized state of the art in medicine, under consideration of currently - available alternative treatments, if any; so that he can achieve an adequate determination or review on the basis of clinical data and thus - if successful - provide sufficient clinical evidence for the medical device at any time. In any case, the clinical evidence obtained must be thorough, objective and conclusive. The clinical evidence obtained must in any case include the following aspects sufficiently specific in terms of the oa. benefit/risk assessment: - Efficacy, especially clinical performance and clinical benefit, each, where possible, by type, extent, duration and probability; - (Clinical) safety, in particular the clinical (residual) risks and adverse reactions (both after adequate minimisation in risk management), - as far as possible, nature, extent, duration and likelihood; - the concrete areas of application, in particular the intended target group(s) and indication(s), including diseases, as far as possible - according to type, severity, stages, forms of progression, phases, etc.), if applicable contraindication(s); - the acceptability of the benefit/risk ratio achieved or the undesirable side effects in comparison with the currently recognised medical - state of the art, - the benefit/risk balance must be compatible with a high level of health and safety protection; and - shall not endanger the clinical condition and safety of patients, or the safety and health of users and, where appropriate, third parties. Further requirements of Annex I may also, on a case-by-case basis, be "candidates" for testing for the need for specific clinical data.
On this basis, the manufacturer establishes the clinical evaluation plan with the following elements: - It shall determine the essential safety and performance requirements set out in Annex I, which shall be supported by relevant clinicaldata: - On this basis, it shall adopt a clinical specification and operationalisation of its data and claims - entirely in accordance with the - above benefit/risk assessment: - on clinical benefit, efficacy, including measurable, patient-relevant outcomes, and - on the qualitative and quantitative parameters for determining clinical safety, including residual risks and undesirable side effects; - the exact target groups and the precise area of application, including indications and contraindications of the MP or an MP designated as equivalent, - criteria for the assessment of the acceptability of the benefit/risk ratio of the MP to the medical state of the art in the field of - application. - A clinical development plan for the planned escalation of clinical trials, from exploratory studies (such as first-in-man; feasibility; pilot; proof-of-concept studies) to confirmatory (pivotal) studies, and - A Post-Market Clinical Follow-up Plan (PMCF Plan) is also required here and must be drawn up particularly carefully for complex, new product developments.
The device description. For additional information, see Appendix A3 (Device description - typical contents) Whether there are any design features of the device, or any indications or target populations, that require specific attention. The clinical evaluation should cover any design features that pose special performance or safety concerns (e.g. presence of medicinal, human or animal components), the intended purpose and application of the device (e.g. target treatment group and disease, proposed warnings, contraindications, precautions,and method of application) and the specific claims made by the manufacturer about the clinical performance and clinical safety of the device. * Information needed for evaluation of equivalence, if equivalence may possibly be claimed. * The risk management documents of the device, e.g. the hazard identification list, clinical risks identified from the risk analysis. The scope of the clinical evaluation will need data from and cross references to the manufacturer’s risk management documents. The risk management documents are expected to identify the risks associated with the device and how such risks have been addressed. The clinical evaluation is expected to address the significance of any clinical risks that remain after design risk mitigation strategies have been employed by the manufacturer. * The current knowledge/ state of the art in the corresponding medical field,such as applicable standards and guidance documents, information relating to the medical condition managed with the device and its naturalcourse, benchmark devices, other devices and medical alternatives available to the target population. * Data source(s) and type(s) of data to be used in the clinical evaluation. * Data relevant to the clinical evaluation may be generated and held by the manufacturer or available from scientific literature. For additional information, see Section 8.1 (Data generated and held by the manufacturer), and Appendix A4 (Sources of literature). * Whether the manufacturer has introduced/ intends to introduce any relevant changes, including - design changes, - changes to materials and manufacturing procedures, - changes to the information materials supplied by the manufacturer (label, IFU, available promotional materials including accompanying documents possibly foreseen by the manufacturer) or other claims, - and whether the claim of equivalence to an existing device is still appropriate. - Whether there are any specific clinical concerns that have newly emerged and need to be addressed. - PMS aspects that need regularly updating in the clinical evaluation report
Risk Management System (RMS)
Based on a risk management plan; the manufacturer must introduce, implement, document and update a risk management system and operate it as a life cycle process under its QMS and systematically keep it up-to-date. This RMS must perform the following: * Define and document a risk management plan for each MD/IVD; * identify known and foreseeable hazards for each product (if possible, by type, severity, probability); * assess and evaluate the risks (if possible, according to type, severity, probability), when used as intended and under reasonably foreseeable misuse; * eliminate or minimise the identified risks in accordance with the risk control principles (see below); * Risks are to be minimized as far as possible, as is possible without negative effects on the benefit/risk ratio ; * Assess the impact of information from the manufacturing phase and PMS on hazards and risks, as well as on the overall benefit/risk ratio and risk acceptance, and * adjust the risk control measures in accordance with the risk control principles on the basis of the above assessment.
The risk control measures for design and manufacture follow these principles:
* they comply with the safety principles, considering the generally recognised state of the art,
* Risk management in risk reduction aims at the acceptability of
* residual risks associated with each hazard, as well as of the
* overall risk
When selecting the most appropriate solutions, the following order of risk control measures must be followed: 1. eliminate or minimise risks as far as possible through safe design and manufacture; 2. if necessary, take appropriate protective measures, including alarms, for risks that cannot be excluded; 3. provide safety information (warnings, cautions, contraindications) and, where appropriate, training for users; manufacturers shall inform users of any residual risks.
To exclude or reduce risks due to use errors, the manufacturer must consider (incl. usability ): * Risks due to ergonomic features of the MD/IVD and the application environment (product design geared to the safety of the patient) and * the technical knowledge, experience, training and further education, if necessary, application environment, health and physical condition of the intended users (laypersons, specialists, handicapped persons or other user-oriented product design).
MDs/IVDs, which are also machines within the meaning of Directive 2006/42/EC, may also have to meet the essential health and safety requirements of Annex I to this Directive. GSPR are specified by harmonised European standards, the references (titles) of which have been published in the Official Journal of the EU for this Regulation (formerly Directives); these standards each contain Annexes Z (ZA, ZB, …) which set out in detail which specific sections of the relevant standard provide a presumption of conformity to which essential requirements of the respective Regulation. Harmonised European standards grant conformity presumptions for certain aspects of the Regulation via these Annexes Z, but they are not binding . Instead, the manufacturer can also fulfil the applicable GSPR and certain other requirements of the Regulation by other means, with detailed reasoning and justification. According to the Regulations, Common Specifications (CS) can now also contribute more detailed presumptions of conformity for certain product groups, especially in the clinical area, or even be binding for Annex XVI products! The lists with the titles of the harmonised European standards which contain presumptions of conformity for one or both Regulations are published 1-2 times a year in the Official Journal of the EU under the heading of the Regulation concerned. The standards themselves are available (in the desired language) from the national standards institutes of the MSs, generally for a fee. Common specifications will be published as Implementing Acts of the COM in the Official Journal of the EU and will also be available via links on the COM website (medical devices sector; see link list).